Lovastatin release from polycaprolactone coated β-tricalcium phosphate: effects of pH, concentration and drug-polymer interactions
- PMID: 23706191
- PMCID: PMC3767773
- DOI: 10.1016/j.msec.2013.02.049
Lovastatin release from polycaprolactone coated β-tricalcium phosphate: effects of pH, concentration and drug-polymer interactions
Abstract
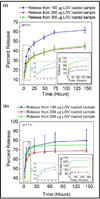
The approach of local drug delivery from polymeric coating is currently getting significant attention for both soft and hard tissue engineering applications for sustained and controlled release. The chemistry of the polymer and the drug, and their interactions influence the release kinetics to a great extent. Here, we examine lovastatin release behaviour from polycaprolactone (PCL) coating on β-tricalcium phosphate (β-TCP). Lovastatin was incorporated into biodegradable water insoluble PCL coating. A burst and uncontrolled lovastatin release was observed from bare β-TCP, whereas controlled and sustained release was observed from PCL coating. A higher lovastatin release was observed pH7.4 as compared to pH5.0. Effect of PCL concentration on lovastatin release was opposite at pH7.4 and 5.0. At pH5.0 lovastatin release was decreased with increasing PCL concentration, whereas release was increased with increasing PCL concentration at pH7.4. High Ca(2+) ion concentration due to high solubility of β-TCP and degradation of PCL coating were observed at pH5.0 compared to no detectable Ca(2+) ion release and visible degradation of PCL coating at pH7.4. The hydrophilic-hydrophobic and hydrophobic-hydrophobic interactions between lovastatin and PCL were found to be the key factors controlling the diffusion dominated release kinetics of lovastatin from PCL coating over dissolution and degradation processes. Understanding the lovastatin release chemistry from PCL will be beneficial for designing drug delivery devices from polymeric coating or scaffolds.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures








References
-
- Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255–270. - PubMed
-
- Ayukawa Y, Yasukawa E, Moriyama Y, Ogino Y, Wada H, Atsuta I, Koyano K. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):336–342. - PubMed
-
- Demierre M-F, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–942. - PubMed
-
- Woo J-T, Nakagawa H, Krecic AM, Nagai K, Hamilton AD, Sebti SM, Stern PH. Inhibitory effects of mevastatin and a geranylgeranyl transferase I inhibitor (GGTI-2166) on mononuclear osteoclast formation induced by receptor activator of NFKB ligand (RANKL) or tumor necrosis factor-α (TNF-α) Biochem Pharmacol. 2005;69(l):87–95. - PubMed
-
- Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–1949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

