Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis
- PMID: 23706739
- PMCID: PMC3983474
- DOI: 10.1016/j.cell.2013.04.032
Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis
Abstract
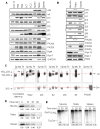
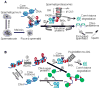
Histone acetylation plays critical roles in chromatin remodeling, DNA repair, and epigenetic regulation of gene expression, but the underlying mechanisms are unclear. Proteasomes usually catalyze ATP- and polyubiquitin-dependent proteolysis. Here, we show that the proteasomes containing the activator PA200 catalyze the polyubiquitin-independent degradation of histones. Most proteasomes in mammalian testes ("spermatoproteasomes") contain a spermatid/sperm-specific α subunit α4 s/PSMA8 and/or the catalytic β subunits of immunoproteasomes in addition to PA200. Deletion of PA200 in mice abolishes acetylation-dependent degradation of somatic core histones during DNA double-strand breaks and delays core histone disappearance in elongated spermatids. Purified PA200 greatly promotes ATP-independent proteasomal degradation of the acetylated core histones, but not polyubiquitinated proteins. Furthermore, acetylation on histones is required for their binding to the bromodomain-like regions in PA200 and its yeast ortholog, Blm10. Thus, PA200/Blm10 specifically targets the core histones for acetylation-mediated degradation by proteasomes, providing mechanisms by which acetylation regulates histone degradation, DNA repair, and spermatogenesis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Proteasome subunit α4s is essential for formation of spermatoproteasomes and histone degradation during meiotic DNA repair in spermatocytes.J Biol Chem. 2021 Jan-Jun;296:100130. doi: 10.1074/jbc.RA120.016485. Epub 2020 Dec 4. J Biol Chem. 2021. PMID: 33262216 Free PMC article.
-
PA200-Mediated Proteasomal Protein Degradation and Regulation of Cellular Senescence.Int J Mol Sci. 2024 May 22;25(11):5637. doi: 10.3390/ijms25115637. Int J Mol Sci. 2024. PMID: 38891826 Free PMC article. Review.
-
The Proteasome Activators Blm10/PA200 Enhance the Proteasomal Degradation of N-Terminal Huntingtin.Biomolecules. 2020 Nov 20;10(11):1581. doi: 10.3390/biom10111581. Biomolecules. 2020. PMID: 33233776 Free PMC article.
-
Cryo-EM structures of the human PA200 and PA200-20S complex reveal regulation of proteasome gate opening and two PA200 apertures.PLoS Biol. 2020 Mar 5;18(3):e3000654. doi: 10.1371/journal.pbio.3000654. eCollection 2020 Mar. PLoS Biol. 2020. PMID: 32134919 Free PMC article.
-
Proteasome activator 200: the heat is on..Mol Cell Proteomics. 2011 May;10(5):R110.006890. doi: 10.1074/mcp.R110.006890. Epub 2011 Mar 9. Mol Cell Proteomics. 2011. PMID: 21389348 Free PMC article. Review.
Cited by
-
Inhibition of Proteasome Activity Induces Formation of Alternative Proteasome Complexes.J Biol Chem. 2016 Jun 17;291(25):13147-59. doi: 10.1074/jbc.M116.717652. Epub 2016 Apr 18. J Biol Chem. 2016. PMID: 27129254 Free PMC article.
-
Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation.Mol Cell Proteomics. 2015 Mar;14(3):658-73. doi: 10.1074/mcp.M114.042812. Epub 2015 Jan 9. Mol Cell Proteomics. 2015. PMID: 25576301 Free PMC article.
-
Phthalates impact on the epigenetic factors contributed specifically by the father at fertilization.Epigenetics Chromatin. 2023 Jan 24;16(1):3. doi: 10.1186/s13072-022-00475-2. Epigenetics Chromatin. 2023. PMID: 36694265 Free PMC article.
-
Aflatoxin levels in maize and peanut and blood in women and children: The case of Timor-Leste.Sci Rep. 2019 Sep 11;9(1):13158. doi: 10.1038/s41598-019-49584-1. Sci Rep. 2019. PMID: 31511633 Free PMC article.
-
The proteasome as a druggable target with multiple therapeutic potentialities: Cutting and non-cutting edges.Pharmacol Ther. 2020 Sep;213:107579. doi: 10.1016/j.pharmthera.2020.107579. Epub 2020 May 19. Pharmacol Ther. 2020. PMID: 32442437 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

