Targeting the viral Achilles' heel: recognition of 5'-triphosphate RNA in innate anti-viral defence
- PMID: 23707340
- PMCID: PMC7185528
- DOI: 10.1016/j.mib.2013.04.009
Targeting the viral Achilles' heel: recognition of 5'-triphosphate RNA in innate anti-viral defence
Abstract
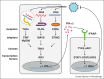
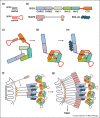
Some RNA virus genomes bear 5'-triphosphates, which can be recognized in the cytoplasm of infected cells by host proteins that mediate anti-viral immunity. Both the innate sensor RIG-I and the interferon-induced IFIT proteins bind to 5'-triphosphate viral RNAs. RIG-I signals for induction of interferons during RNA virus infection while IFITs sequester viral RNAs to exert an anti-viral effect. Notably, the structures of these proteins reveal both similarities and differences, which are suggestive of independent evolution towards ligand binding. 5'-triphosphates, which are absent from most RNAs in the cytosol of uninfected cells, are thus a marker of virus infection that is targeted by the innate immune system for both induction and execution of the anti-viral response.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. - PubMed
-
- Pestka S., Krause C.D., Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
-
- Pichlmair A., Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. - PubMed
-
- Crow Y.J. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 2011;1238:91–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

