Validation of cryo-EM structure of IP₃R1 channel
- PMID: 23707684
- PMCID: PMC3696195
- DOI: 10.1016/j.str.2013.04.016
Validation of cryo-EM structure of IP₃R1 channel
Abstract
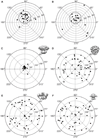
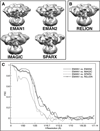
About a decade ago, three electron cryomicroscopy (cryo-EM) single-particle reconstructions of IP3R1 were reported at low resolution. It was disturbing that these structures bore little similarity to one another, even at the level of quaternary structure. Recently, we published an improved structure of IP3R1 at ∼1 nm resolution. However, this structure did not bear any resemblance to any of the three previously published structures, leading to the question of why the structure should be considered more reliable than the original three. Here, we apply several methods, including class-average/map comparisons, tilt-pair validation, and use of multiple refinement software packages, to give strong evidence for the reliability of our recent structure. The map resolution and feature resolvability are assessed with the gold standard criterion. This approach is generally applicable to assessing the validity of cryo-EM maps of other molecular machines.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Chen DH, Song JL, Chuang DT, Chiu W, Ludtke SJ. An expanded conformation of single-ring GroEL-GroES complex encapsulates an 86 kDa substrate. Structure. 2006;14:1711–1722. - PubMed
-
- Hamada K, Terauchi A, Mikoshiba K. Three-dimensional rearrangements within inositol 1, 4, 5-trisphosphate receptor by calcium. J Biol Chem. 2003;278:52881–52889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

