The microRNA-200 family targets multiple non-small cell lung cancer prognostic markers in H1299 cells and BEAS-2B cells
- PMID: 23708087
- PMCID: PMC3775564
- DOI: 10.3892/ijo.2013.1963
The microRNA-200 family targets multiple non-small cell lung cancer prognostic markers in H1299 cells and BEAS-2B cells
Abstract
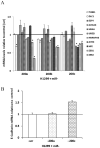
Lung cancer remains the leading cause of cancer-related mortality for both men and women. Tumor recurrence and metastasis is the major cause of lung cancer treatment failure and death. The microRNA‑200 (miR-200) family is a powerful regulator of the epithelial-mesenchymal transition (EMT) process, which is essential in tumor metastasis. Nevertheless, miR-200 family target genes that promote metastasis in non-small cell lung cancer (NSCLC) remain largely unknown. Here, we sought to investigate whether the microRNA-200 family regulates our previously identified NSCLC prognostic marker genes associated with metastasis, as potential molecular targets. Novel miRNA targets were predicted using bioinformatics tools based on correlation analyses of miRNA and mRNA expression in 57 squamous cell lung cancer tumor samples. The predicted target genes were validated with quantitative RT-PCR assays and western blot analysis following re-expression of miR-200a, -200b and -200c in the metastatic NSCLC H1299 cell line. The results show that restoring miR-200a or miR-200c in H1299 cells induces downregulation of DLC1, ATRX and HFE. Reinforced miR-200b expression results in downregulation of DLC1, HNRNPA3 and HFE. Additionally, miR-200 family downregulates HNRNPR3, HFE and ATRX in BEAS-2B immortalized lung epithelial cells in quantitative RT-PCR and western blot assays. The miR-200 family and these potential targets are functionally involved in canonical pathways of immune response, molecular mechanisms of cancer, metastasis signaling, cell-cell communication, proliferation and DNA repair in Ingenuity pathway analysis (IPA). These results indicate that re-expression of miR-200 downregulates our previously identified NSCLC prognostic biomarkers in metastatic NSCLC cells. These results provide new insights into miR-200 regulation in lung cancer metastasis and consequent clinical outcome, and may provide a potential basis for innovative therapeutic approaches for the treatment of this deadly disease.
Figures





References
-
- Monteiro J, Fodde R. Cancer stemness and metastasis: therapeutic consequences and perspectives. Eur J Cancer. 2010;46:1198–1203. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
-
- Dohadwala M, Yang SC, Luo J, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–5345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

