Asymmetric palladium-catalysed intramolecular Wacker-type cyclisations of unsaturated alcohols and amino alcohols
- PMID: 23708231
- PMCID: PMC6270647
- DOI: 10.3390/molecules18066173
Asymmetric palladium-catalysed intramolecular Wacker-type cyclisations of unsaturated alcohols and amino alcohols
Abstract
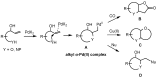
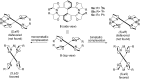
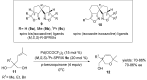
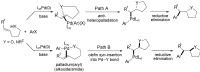
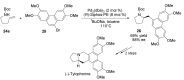
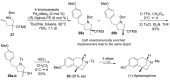
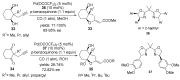
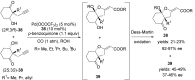
The palladium (II)-catalysed reactions of alkenols and aminoalkenols such as oxycarbonylations or bicyclisations are powerful methods for the construction of oxygen and nitrogen-containing heterocyclic compounds. This review highlights recent progress in the development of the asymmetric palladium(II)-catalysed Wacker-type cyclisations of unsaturated polyols and aminoalcohols. The scope, limitations, and applications of these reactions are presented.
Figures
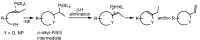
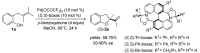

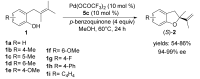
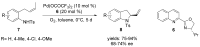


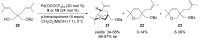



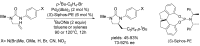


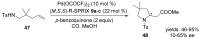
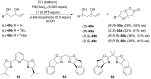

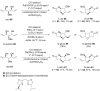
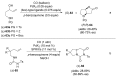
References
-
- Tsuji J. Palladium Reagents and Catalysis. John Wiley & Sons Ltd.; Chichester, UK/Hoboken, NJ, USA: 2004.
-
- Malleron J.-L., Fiaud J.-C., Legros J.-Y. Handbook of Palladium-Catalyzed Organic Reactions. Academic Press; London, UK: 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

