Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination
- PMID: 23708355
- PMCID: PMC3660628
- DOI: 10.1016/j.bpj.2013.03.058
Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination
Abstract
The factors responsible for the regulation of regenerative calcium-induced calcium release (CICR) during Ca(2+) spark evolution remain unclear. Cardiac ryanodine receptor (RyR) gating in rats and sheep was recorded at physiological Ca(2+), Mg(2+), and ATP levels and incorporated into a 3D model of the cardiac dyad, which reproduced the time course of Ca(2+) sparks, Ca(2+) blinks, and Ca(2+) spark restitution. The termination of CICR by induction decay in the model principally arose from the steep Ca(2+) dependence of RyR closed time, with the measured sarcoplasmic reticulum (SR) lumen Ca(2+) dependence of RyR gating making almost no contribution. The start of CICR termination was strongly dependent on the extent of local depletion of junctional SR Ca(2+), as well as the time course of local Ca(2+) gradients within the junctional space. Reducing the dimensions of the dyad junction reduced Ca(2+) spark amplitude by reducing the strength of regenerative feedback within CICR. A refractory period for Ca(2+) spark initiation and subsequent Ca(2+) spark amplitude restitution arose from 1), the extent to which the regenerative phase of CICR can be supported by the partially depleted junctional SR, and 2), the availability of releasable Ca(2+) in the junctional SR. The physical organization of RyRs within the junctional space had minimal effects on Ca(2+) spark amplitude when more than nine RyRs were present. Spark amplitude had a nonlinear dependence on RyR single-channel Ca(2+) flux, and was approximately halved by reducing the flux from 0.6 to 0.2 pA. Although rat and sheep RyRs had quite different Ca(2+) sensitivities, Ca(2+) spark amplitude was hardly affected. This suggests that moderate changes in RyR gating by second-messenger systems will principally alter the spatiotemporal properties of SR release, with smaller effects on the amount released.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


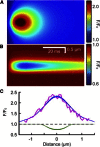


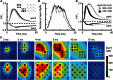

Comment in
-
Extinguishing the sparks.Biophys J. 2013 May 21;104(10):2115-7. doi: 10.1016/j.bpj.2013.04.010. Biophys J. 2013. PMID: 23708349 Free PMC article. No abstract available.
Similar articles
-
Termination of calcium-induced calcium release by induction decay: an emergent property of stochastic channel gating and molecular scale architecture.J Mol Cell Cardiol. 2013 Jan;54:98-100. doi: 10.1016/j.yjmcc.2012.10.009. Epub 2012 Nov 1. J Mol Cell Cardiol. 2013. PMID: 23123322
-
Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle.Circ Res. 2012 Jun 22;111(1):28-36. doi: 10.1161/CIRCRESAHA.112.265652. Epub 2012 May 24. Circ Res. 2012. PMID: 22628577 Free PMC article.
-
Cytosolic Ca²⁺ buffering determines the intra-SR Ca²⁺ concentration at which cardiac Ca²⁺ sparks terminate.Cell Calcium. 2015 Sep;58(3):246-53. doi: 10.1016/j.ceca.2015.06.002. Epub 2015 Jun 10. Cell Calcium. 2015. PMID: 26095947 Free PMC article.
-
Pernicious attrition and inter-RyR2 CICR current control in cardiac muscle.J Mol Cell Cardiol. 2013 May;58:53-8. doi: 10.1016/j.yjmcc.2013.01.011. Epub 2013 Jan 28. J Mol Cell Cardiol. 2013. PMID: 23369697 Free PMC article. Review.
-
Calcium signaling between sarcolemmal calcium channels and ryanodine receptors in heart cells.Front Biosci. 2002 Sep 1;7:d1867-78. doi: 10.2741/A885. Front Biosci. 2002. PMID: 12161336 Review.
Cited by
-
Computational modeling of subcellular transport and signaling.Curr Opin Struct Biol. 2014 Apr;25:92-7. doi: 10.1016/j.sbi.2014.01.006. Epub 2014 Feb 7. Curr Opin Struct Biol. 2014. PMID: 24509246 Free PMC article. Review.
-
Local recovery of cardiac calcium-induced calcium release interrogated by ultra-effective, two-photon uncaging of calcium.J Physiol. 2021 Aug;599(16):3841-3852. doi: 10.1113/JP281482. Epub 2021 Aug 2. J Physiol. 2021. PMID: 34245001 Free PMC article.
-
Estimating the probabilities of rare arrhythmic events in multiscale computational models of cardiac cells and tissue.PLoS Comput Biol. 2017 Nov 16;13(11):e1005783. doi: 10.1371/journal.pcbi.1005783. eCollection 2017 Nov. PLoS Comput Biol. 2017. PMID: 29145393 Free PMC article.
-
On the Adjacency Matrix of RyR2 Cluster Structures.PLoS Comput Biol. 2015 Nov 6;11(11):e1004521. doi: 10.1371/journal.pcbi.1004521. eCollection 2015 Nov. PLoS Comput Biol. 2015. PMID: 26545234 Free PMC article.
-
Ambiguous interactions between diastolic and SR Ca2+ in the regulation of cardiac Ca2+ release.J Gen Physiol. 2017 Sep 4;149(9):847-855. doi: 10.1085/jgp.201711814. Epub 2017 Aug 10. J Gen Physiol. 2017. PMID: 28798276 Free PMC article. Review.
References
-
- Bers D.M. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Meissner G., Rousseau E., Anderson K.A. Biochemical characterization of the Ca2+ release channel of skeletal and cardiac sarcoplasmic reticulum. Mol. Cell. Biochem. 1988;82:59–65. - PubMed
-
- Inui M., Fleischer S. Purification of Ca2+ release channel (ryanodine receptor) from heart and skeletal muscle sarcoplasmic reticulum. Methods Enzymol. 1988;157:490–505. - PubMed
-
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245:C1–C14. - PubMed
-
- Cannell M.B., Berlin J.R., Lederer W.J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987;238:1419–1423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

