Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial
- PMID: 23708466
- PMCID: PMC3918120
- DOI: 10.1007/s00787-013-0421-y
Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial
Abstract
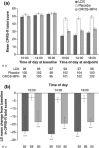
Lisdexamfetamine dimesylate (LDX) is a long-acting, prodrug stimulant therapy for patients with attention-deficit/hyperactivity disorder (ADHD). This randomized placebo-controlled trial of an optimized daily dose of LDX (30, 50 or 70 mg) was conducted in children and adolescents (aged 6-17 years) with ADHD. To evaluate the efficacy of LDX throughout the day, symptoms and behaviors of ADHD were evaluated using an abbreviated version of the Conners' Parent Rating Scale-Revised (CPRS-R) at 1000, 1400 and 1800 hours following early morning dosing (0700 hours). Osmotic-release oral system methylphenidate (OROS-MPH) was included as a reference treatment, but the study was not designed to support a statistical comparison between LDX and OROS-MPH. The full analysis set comprised 317 patients (LDX, n = 104; placebo, n = 106; OROS-MPH, n = 107). At baseline, CPRS-R total scores were similar across treatment groups. At endpoint, differences (active treatment - placebo) in least squares (LS) mean change from baseline CPRS-R total scores were statistically significant (P < 0.001) throughout the day for LDX (effect sizes: 1000 hours, 1.42; 1400 hours, 1.41; 1800 hours, 1.30) and OROS-MPH (effect sizes: 1000 hours, 1.04; 1400 hours, 0.98; 1800 hours, 0.92). Differences in LS mean change from baseline to endpoint were statistically significant (P < 0.001) for both active treatments in all four subscales of the CPRS-R (ADHD index, oppositional, hyperactivity and cognitive). In conclusion, improvements relative to placebo in ADHD-related symptoms and behaviors in children and adolescents receiving a single morning dose of LDX or OROS-MPH were maintained throughout the day and were ongoing at the last measurement in the evening (1800 hours).
Figures

References
-
- World Health Organization (2004) F90 Hyperkinetic disorders. In: ICD-10: International statistical classification of diseases and related health problems, 2nd edn. 10th Revision. World Health Organization, Geneva
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

