Dihydroartemisinin promotes angiogenesis during the early embryonic development of zebrafish
- PMID: 23708556
- PMCID: PMC4003027
- DOI: 10.1038/aps.2013.48
Dihydroartemisinin promotes angiogenesis during the early embryonic development of zebrafish
Abstract
Aim: To investigate the embryotoxicity of dihydroartemisinin (DHA), the main active metabolite of artemisinin, in zebrafish, and explore the corresponding mechanisms.
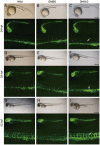
Methods: The embryos of wild type and TG (flk1:GFP) transgenic zebrafish were exposed to DHA. Developmental phenotypes of the embryos were observed. Development of blood vessels was directly observed in living embryos of TG (flk1:GFP) transgenic zebrafish under fluorescence microscope. The expression of angiogenesis marker genes vegfa, flk1, and flt1 in the embryos was detected using real-time PCR and RNA in situ hybridization assays.
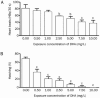
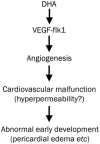
Results: Exposure to DHA (1-10 mg/L) dose-dependently caused abnormal zebrafish embryonic phenotypes in the early developmental stage. Furthermore, exposure to DHA (10 mg/L) resulted in more pronounced embryonic angiogenesis in TG (flk1:GFP) zebrafish line. Exposure to DHA (10 mg/L) significantly increased the mRNA expression of vegfa, flk1, and flt1 in the embryos. Knockdown of the flk1 protein partially blocked the effects of DHA on embryogenesis.
Conclusion: DHA causes abnormal embryonic phenotypes and promotes angiogenesis in zebrafish early embryonic development, demonstrating the potential embryotoxicity of DHA.
Figures



References
-
- Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–55. - PubMed
-
- Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med. 2009;11:e32. - PubMed
-
- Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

