Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis
- PMID: 23708660
- PMCID: PMC4221191
- DOI: 10.1038/onc.2013.157
Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis
Abstract
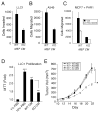
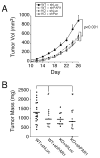
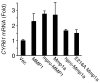
Matrix metalloprotease-1 (MMP1) is an important mediator of tumorigenesis, inflammation and tissue remodeling through its ability to degrade critical matrix components. Recent studies indicate that stromal-derived MMP1 may exert direct oncogenic activity by signaling through protease-activated receptor-1 (PAR1) in carcinoma cells; however, this has not been established in vivo. We generated an Mmp1a knockout mouse to ascertain whether stromal-derived Mmp1a affects tumor growth. Mmp1a-deficient mice are grossly normal and born in Mendelian ratios; however, deficiency of Mmp1a results in significantly decreased growth and angiogenesis of lung tumors. Coimplantation of lung cancer cells with wild-type Mmp1a(+/+) fibroblasts completely restored tumor growth in Mmp1a-deficient animals, highlighting the critical role of stromal-derived Mmp1a. Silencing of PAR1 expression in the lung carcinoma cells phenocopied stromal Mmp1a-deficiency, thus validating tumor-derived PAR1 as an Mmp1a target. Mmp1a secretion is controlled by the ability of its prodomain to facilitate autocleavage, whereas human MMP1 is efficiently secreted because of stable pro- and catalytic domain interactions. Taken together, these data demonstrate that stromal Mmp1a drives in vivo tumorigenesis and provide proof of concept that targeting the MMP1-PAR1 axis may afford effective treatments of lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
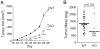


References
-
- Kanamori Y, Matsushima M, Minaguchi T, Kobayashi K, Sagae S, Kudo R, et al. Correlation between expression of the matrix metalloproteinase-1 gene in ovarian cancers and an insertion/deletion polymorphism in its promoter region. Cancer Research. 1999;59:4225–4227. - PubMed
-
- Nikkola J, Vihinen P, Vlaykova T, Hahka-Kemppinen M, Kähäri V-M, Pyrhönen S. High expression levels of collagenase-1 and stromelysin-1 correlate with shorter disease-free survival in human metastatic melanoma. Int J Cancer. 2002;97:432–438. - PubMed
-
- Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

