High-fat feeding-induced hyperinsulinemia increases cardiac glucose uptake and mitochondrial function despite peripheral insulin resistance
- PMID: 23709089
- PMCID: PMC5398492
- DOI: 10.1210/en.2012-2272
High-fat feeding-induced hyperinsulinemia increases cardiac glucose uptake and mitochondrial function despite peripheral insulin resistance
Abstract
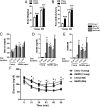
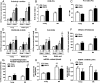
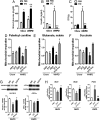
In obesity, reduced cardiac glucose uptake and mitochondrial abnormalities are putative causes of cardiac dysfunction. However, high-fat diet (HFD) does not consistently induce cardiac insulin resistance and mitochondrial damage, and recent studies suggest HFD may be cardioprotective. To determine cardiac responses to HFD, we investigated cardiac function, glucose uptake, and mitochondrial respiration in young (3-month-old) and middle-aged (MA) (12-month-old) male Ldlr(-/-) mice fed chow or 3 months HFD to induce obesity, systemic insulin resistance, and hyperinsulinemia. In MA Ldlr(-/-) mice, HFD induced accelerated atherosclerosis and nonalcoholic steatohepatitis, common complications of human obesity. Surprisingly, HFD-fed mice demonstrated increased cardiac glucose uptake, which was most prominent in MA mice, in the absence of cardiac contractile dysfunction or hypertrophy. Moreover, hearts of HFD-fed mice had enhanced mitochondrial oxidation of palmitoyl carnitine, glutamate, and succinate and greater basal insulin signaling compared with those of chow-fed mice, suggesting cardiac insulin sensitivity was maintained, despite systemic insulin resistance. Streptozotocin-induced ablation of insulin production markedly reduced cardiac glucose uptake and mitochondrial dysfunction in HFD-fed, but not in chow-fed, mice. Insulin injection reversed these effects, suggesting that insulin may protect cardiac mitochondria during HFD. These results have implications for cardiac metabolism and preservation of mitochondrial function in obesity.
Figures




Comment in
-
Cardiac insulin resistance: it's sweeter than you think.Endocrinology. 2013 Aug;154(8):2575-8. doi: 10.1210/en.2013-1506. Endocrinology. 2013. PMID: 23873770 No abstract available.
References
-
- Ouwens DM, Boer C, Fodor M et al. . Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48:1229–1237. - PubMed
-
- Utriainen T, Takala T, Luotolahti M et al. . Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia. 1998;41:555–559. - PubMed
-
- Iozzo P, Chareonthaitawee P, Dutka D et al. . Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–3024. - PubMed
-
- Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res. 2011;89:148–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

