A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma
- PMID: 23709298
- PMCID: PMC4211292
- DOI: 10.1158/1541-7786.MCR-13-0111
A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma
Abstract
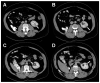
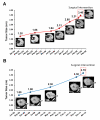
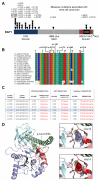
Renal cell carcinoma (RCC) clusters in some families. Familial RCC arises from mutations in several genes, including the von Hippel-Lindau (VHL) tumor suppressor, which is also mutated in sporadic RCC. However, a significant percentage of familial RCC remains unexplained. Recently, we discovered that the BRCA1-associated protein-1 (BAP1) gene is mutated in sporadic RCC. The BAP1 gene encodes a nuclear deubiquitinase and appears to be a classic two-hit tumor suppressor gene. Somatic BAP1 mutations are associated with high-grade, clear-cell RCC (ccRCC) and poor patient outcomes. To determine whether BAP1 predisposes to familial RCC, the BAP1 gene was sequenced in 83 unrelated probands with unexplained familial RCC. Interestingly, a novel variant (c.41T>A; p.L14H) was uncovered that cosegregated with the RCC phenotype. The p.L14H variant targets a highly conserved residue in the catalytic domain, which is frequently targeted by missense mutations. The family with the novel BAP1 variant was characterized by early-onset ccRCC, occasionally of high Fuhrman grade, and lacked other features that typify VHL syndrome. These findings suggest that BAP1 is an early-onset familial RCC predisposing gene.
Implications: BAP1 mutations may drive tumor development in a subset of patients with inherited renal cell cancer.
©2013 AACR.
Figures




References
-
- Pavlovich CP, Schmidt LS. Searching for the hereditary causes of renal-cell carcinoma. Nat Rev Cancer. 2004;4:381–93. - PubMed
-
- Malinoc A, Sullivan M, Wiech T, Kurt Werner S, Jilg C, Straeter J, et al. Biallelic inactivation of the SDHC gene in renal carcinoma associated with paraganglioma syndrome type 3. Endo Relat Ca. 2012;19:283–90. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

