FAMP, a novel apoA-I mimetic peptide, suppresses aortic plaque formation through promotion of biological HDL function in ApoE-deficient mice
- PMID: 23709562
- PMCID: PMC3698760
- DOI: 10.1161/JAHA.113.000048
FAMP, a novel apoA-I mimetic peptide, suppresses aortic plaque formation through promotion of biological HDL function in ApoE-deficient mice
Abstract
Background: Apolipoprotein (apo) A-I is a major high-density lipoprotein (HDL) protein that causes cholesterol efflux from peripheral cells through the ATP-binding cassette transporter A1 (ABCA1), thus generating HDL and reversing the macrophage foam cell phenotype. Pre-β1 HDL is the smallest subfraction of HDL, which is believed to represent newly formed HDL, and it is the most active acceptor of free cholesterol. Furthermore it has a possible protective function against cardiovascular disease (CVD). We developed a novel apoA-I mimetic peptide without phospholipids (Fukuoka University ApoA-I Mimetic Peptide, FAMP).
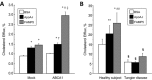
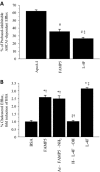
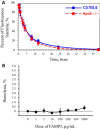
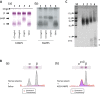
Methods and results: FAMP type 5 (FAMP5) had a high capacity for cholesterol efflux from A172 cells and mouse and human macrophages in vitro, and the efflux was mainly dependent on ABCA1 transporter. Incubation of FAMP5 with human HDL or whole plasma generated small HDL particles, and charged apoA-I-rich particles migrated as pre-β HDL on agarose gel electrophoresis. Sixteen weeks of treatment with FAMP5 significantly suppressed aortic plaque formation (scrambled FAMP, 31.3 ± 8.9% versus high-dose FAMP5, 16.2 ± 5.0%; P<0.01) and plasma C-reactive protein and monocyte chemoattractant protein-1 in apoE-deficient mice fed a high-fat diet. In addition, it significantly enhanced HDL-mediated cholesterol efflux capacity from the mice.
Conclusions: A newly developed apoA-I mimetic peptide, FAMP, has an antiatherosclerotic effect through the enhancement of the biological function of HDL. FAMP may have significant atheroprotective potential and prove to be a new therapeutic tool for CVD.
Keywords: ATP‐binding cassette transporters; HDL particle size; apolipoproteins; peptides; pre‐β HDL.
Figures





Similar articles
-
High-density lipoprotein and atherosclerosis: Roles of lipid transporters.World J Cardiol. 2014 Oct 26;6(10):1049-59. doi: 10.4330/wjc.v6.i10.1049. World J Cardiol. 2014. PMID: 25349649 Free PMC article. Review.
-
Anti-atherosclerotic effects of an improved apolipoprotein A-I mimetic peptide.Int J Cardiol. 2019 Dec 15;297:111-117. doi: 10.1016/j.ijcard.2019.08.043. Epub 2019 Aug 22. Int J Cardiol. 2019. PMID: 31519377
-
HDL and CER-001 Inverse-Dose Dependent Inhibition of Atherosclerotic Plaque Formation in apoE-/- Mice: Evidence of ABCA1 Down-Regulation.PLoS One. 2015 Sep 3;10(9):e0137584. doi: 10.1371/journal.pone.0137584. eCollection 2015. PLoS One. 2015. PMID: 26335690 Free PMC article.
-
Newly developed apolipoprotein A-I mimetic peptide promotes macrophage reverse cholesterol transport in vivo.Int J Cardiol. 2015 Aug 1;192:82-8. doi: 10.1016/j.ijcard.2015.05.012. Epub 2015 May 7. Int J Cardiol. 2015. PMID: 26005953
-
High-Density Lipoprotein Mimetics: a Therapeutic Tool for Atherosclerotic Diseases.J Atheroscler Thromb. 2016;23(4):385-94. doi: 10.5551/jat.33720. Epub 2016 Feb 1. J Atheroscler Thromb. 2016. PMID: 26830201 Review.
Cited by
-
HDL Mimetic Peptides.Adv Exp Med Biol. 2022;1377:141-151. doi: 10.1007/978-981-19-1592-5_11. Adv Exp Med Biol. 2022. PMID: 35575927
-
High-density lipoprotein mimetics: promises and challenges.Biochem J. 2015 Dec 15;472(3):249-59. doi: 10.1042/BJ20150832. Biochem J. 2015. PMID: 26613945 Free PMC article. Review.
-
Apolipoprotein Mimetic Peptides: Potential New Therapies for Cardiovascular Diseases.Cells. 2021 Mar 8;10(3):597. doi: 10.3390/cells10030597. Cells. 2021. PMID: 33800446 Free PMC article. Review.
-
A novel apoA-I mimetic peptide suppresses atherosclerosis by promoting physiological HDL function in apoE-/- mice.Br J Pharmacol. 2020 Oct;177(20):4627-4644. doi: 10.1111/bph.15213. Epub 2020 Sep 9. Br J Pharmacol. 2020. PMID: 32726461 Free PMC article.
-
High-density lipoprotein and atherosclerosis: Roles of lipid transporters.World J Cardiol. 2014 Oct 26;6(10):1049-59. doi: 10.4330/wjc.v6.i10.1049. World J Cardiol. 2014. PMID: 25349649 Free PMC article. Review.
References
-
- von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001; 21:13-27 - PubMed
-
- Yancey PG, Bortnick AE, Kellner‐Weibel G, de la Llera‐Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003; 23:712-719 - PubMed
-
- Davidson WS, Sparks DL, Lund‐Katz S, Phillips MC. The molecular basis for the difference in charge between pre‐beta‐ and alpha‐migrating high density lipoproteins. J Biol Chem. 1994; 269:8959-8965 - PubMed
-
- Kunitake ST, La Sala KJ, Kane JP. Apolipoprotein A‐I‐containing lipoproteins with pre‐beta electrophoretic mobility. J Lipid Res. 1985; 26:549-555 - PubMed
-
- Castro GR, Fielding CJ. Early incorporation of cell‐derived cholesterol into pre‐beta‐migrating high‐density lipoprotein. Biochemistry. 1988; 27:25-29 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

