Standardization and cross validation of alloreactive IFNγ ELISPOT assays within the clinical trials in organ transplantation consortium
- PMID: 23710568
- PMCID: PMC3839289
- DOI: 10.1111/ajt.12286
Standardization and cross validation of alloreactive IFNγ ELISPOT assays within the clinical trials in organ transplantation consortium
Abstract
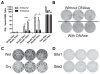
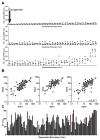
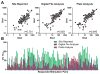
Emerging evidence indicates memory donor-reactive T cells are detrimental to transplant outcome and that quantifying the frequency of IFNγ-producing, donor-reactive PBMCs by ELISPOT has potential utility as an immune monitoring tool. Nonetheless, differences in assay performance among laboratories limit the ability to compare results. In an effort to standardize assays, we prepared a panel of common cellular reagent standards, developed and cross validated a standard operating procedure (SOP) for alloreactive IFNγ ELISPOT assays in several research laboratories supported by the NIH-funded Clinical Trials in Organ Transplantation (CTOT) Consortium. We demonstrate that strict adherence to the SOP and centralized data analysis results in high reproducibility with a coefficient of variance (CV) of ≈ 30%. This standardization of IFNγ ELISPOT assay will facilitate interpretation of data from multicenter transplantation research studies and provide the foundation for developing clinical laboratory testing strategies to guide therapeutic decision-making in transplant patients.
© Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures

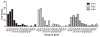

References
-
- Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011 Mar;11(3):450–62. - PubMed
-
- Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011 Jun;11(6):1226–35. - PubMed
-
- Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004 Aug;4(8):1289–95. - PubMed
-
- Meyers BF, de la Morena M, Sweet SC, Trulock EP, Guthrie TJ, Mendeloff EN, et al. Primary graft dysfunction and other selected complications of lung transplantation: A single-center experience of 983 patients. J Thorac Cardiovasc Surg. 2005 Jun;129(6):1421–9. - PubMed
-
- Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med. 1994 Aug 11;331(6):365–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

