Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation
- PMID: 23710605
- PMCID: PMC3706882
- DOI: 10.1186/scrt209
Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation
Abstract
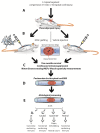
Introduction: Intraspinal grafting of human neural stem cells represents a promising approach to promote recovery of function after spinal trauma. Such a treatment may serve to: I) provide trophic support to improve survival of host neurons; II) improve the structural integrity of the spinal parenchyma by reducing syringomyelia and scarring in trauma-injured regions; and III) provide neuronal populations to potentially form relays with host axons, segmental interneurons, and/or α-motoneurons. Here we characterized the effect of intraspinal grafting of clinical grade human fetal spinal cord-derived neural stem cells (HSSC) on the recovery of neurological function in a rat model of acute lumbar (L3) compression injury.
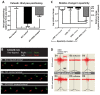
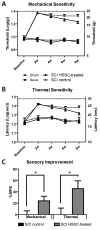
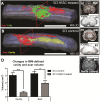
Methods: Three-month-old female Sprague-Dawley rats received L3 spinal compression injury. Three days post-injury, animals were randomized and received intraspinal injections of either HSSC, media-only, or no injections. All animals were immunosuppressed with tacrolimus, mycophenolate mofetil, and methylprednisolone acetate from the day of cell grafting and survived for eight weeks. Motor and sensory dysfunction were periodically assessed using open field locomotion scoring, thermal/tactile pain/escape thresholds and myogenic motor evoked potentials. The presence of spasticity was measured by gastrocnemius muscle resistance and electromyography response during computer-controlled ankle rotation. At the end-point, gait (CatWalk), ladder climbing, and single frame analyses were also assessed. Syrinx size, spinal cord dimensions, and extent of scarring were measured by magnetic resonance imaging. Differentiation and integration of grafted cells in the host tissue were validated with immunofluorescence staining using human-specific antibodies.
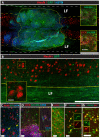
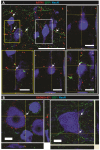
Results: Intraspinal grafting of HSSC led to a progressive and significant improvement in lower extremity paw placement, amelioration of spasticity, and normalization in thermal and tactile pain/escape thresholds at eight weeks post-grafting. No significant differences were detected in other CatWalk parameters, motor evoked potentials, open field locomotor (Basso, Beattie, and Bresnahan locomotion score (BBB)) score or ladder climbing test. Magnetic resonance imaging volume reconstruction and immunofluorescence analysis of grafted cell survival showed near complete injury-cavity-filling by grafted cells and development of putative GABA-ergic synapses between grafted and host neurons.
Conclusions: Peri-acute intraspinal grafting of HSSC can represent an effective therapy which ameliorates motor and sensory deficits after traumatic spinal cord injury.
Figures

Comment in
-
Human neural stem cell transplantation in spinal cord injury models: how far from clinical application?Stem Cell Res Ther. 2013 Jun 13;4(3):61. doi: 10.1186/scrt210. Stem Cell Res Ther. 2013. PMID: 23768980 Free PMC article.
Similar articles
-
Analysis of dosing regimen and reproducibility of intraspinal grafting of human spinal stem cells in immunosuppressed minipigs.Cell Transplant. 2010;19(9):1103-22. doi: 10.3727/096368910X503406. Epub 2010 Apr 21. Cell Transplant. 2010. PMID: 20412634
-
Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells.Neuroscience. 2007 Jun 29;147(2):546-60. doi: 10.1016/j.neuroscience.2007.02.065. Epub 2007 May 23. Neuroscience. 2007. PMID: 17524565 Free PMC article.
-
Survival and differentiation of human embryonic stem cell-derived neural precursors grafted spinally in spinal ischemia-injured rats or in naive immunosuppressed minipigs: a qualitative and quantitative study.Cell Transplant. 2012;21(12):2603-19. doi: 10.3727/096368912X653200. Epub 2012 Aug 10. Cell Transplant. 2012. PMID: 22889456
-
Repair of spinal cord injury with neuronal relays: From fetal grafts to neural stem cells.Brain Res. 2015 Sep 4;1619:115-23. doi: 10.1016/j.brainres.2015.01.006. Epub 2015 Jan 12. Brain Res. 2015. PMID: 25591483 Free PMC article. Review.
-
The role of embryonic motoneuron transplants to restore the lost motor function of the injured spinal cord.Ann Anat. 2011 Jul;193(4):362-70. doi: 10.1016/j.aanat.2011.04.001. Epub 2011 Apr 30. Ann Anat. 2011. PMID: 21600746 Review.
Cited by
-
T cell mediated suppression of neurotropic coronavirus replication in neural precursor cells.Virology. 2014 Jan 20;449:235-43. doi: 10.1016/j.virol.2013.11.025. Epub 2013 Dec 12. Virology. 2014. PMID: 24418558 Free PMC article.
-
A New Acute Impact-Compression Lumbar Spinal Cord Injury Model in the Rodent.J Neurotrauma. 2016 Feb 1;33(3):278-89. doi: 10.1089/neu.2015.3937. Epub 2015 Dec 1. J Neurotrauma. 2016. PMID: 26414192 Free PMC article.
-
Long-term clinical and safety outcomes from a single-site phase 1 study of neural stem cell transplantation for chronic thoracic spinal cord injury.Cell Rep Med. 2024 Dec 17;5(12):101841. doi: 10.1016/j.xcrm.2024.101841. Epub 2024 Dec 2. Cell Rep Med. 2024. PMID: 39626671 Free PMC article. Clinical Trial.
-
Evaluation of spinal cord injury animal models.Neural Regen Res. 2014 Nov 15;9(22):2008-12. doi: 10.4103/1673-5374.143436. Neural Regen Res. 2014. PMID: 25598784 Free PMC article.
-
Stem cells: A time to heal.Nature. 2013 Nov 14;503(7475):S4-6. doi: 10.1038/503S4a. Nature. 2013. PMID: 24227005 No abstract available.
References
-
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

