Anti-vascular endothelial growth factor antibody attenuates inflammation and decreases mortality in an experimental model of severe sepsis
- PMID: 23710641
- PMCID: PMC4056034
- DOI: 10.1186/cc12742
Anti-vascular endothelial growth factor antibody attenuates inflammation and decreases mortality in an experimental model of severe sepsis
Abstract
Introduction: Severe sepsis is associated with an unacceptably high rate of mortality. Recent studies revealed elevated levels of vascular endothelial growth factor (VEGF), a potent angiogenic and vascular permeability factor, in patients with sepsis. There was also an association between VEGF levels and sepsis severity. Here we investigate the effects of an anti-VEGF antibody (Bevacizumab, Bev) in an experimental model of sepsis.
Methods: Human umbilical vein endothelial cells (HUVECs), murine cecal ligation and puncture (CLP), and endotoxemia models of sepsis were used. HUVECs were treated with lipopolysaccharide (LPS) and/or Bev, harvested and cytokine mRNA levels determined using a semi-quantitative reverse transcription-polymerase chain reaction assay. The levels of inflammatory cytokine were also determined in HUVECs supernatants. In addition, the effects of Bev on mortality in the CLP and endotoxemia models of sepsis were evaluated.
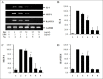
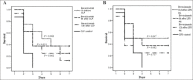
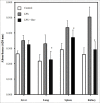
Results: Treatment with Bev and LPS significantly decreased the expression and the level of inflammatory cytokines in HUVECs relative to LPS alone. In CLP and endotoxemia models, survival benefits were evident in mice given 0.1 mg/kg of Bev relative to the CLP or LPS alone (P<0.001 and P=0.028, respectively), and in 6 h post-treated mice relative to the CLP alone for the effect of different time of Bev (P=0.033). In addition, Bev treatment inhibited LPS-induced vascular leak in the lung, spleen and kidney in the murine endotoxemia model (P<0.05).
Conclusions: Anti-VEGF antibody may be a promising therapeutic agent due to its beneficial effects on the survival of sepsis by decreasing inflammatory responses and endothelial permeability.
Figures


Comment in
-
Does anti-VEGF bevacizumab improve survival in experimental sepsis?Crit Care. 2017 Jul 5;21(1):163. doi: 10.1186/s13054-017-1734-x. Crit Care. 2017. PMID: 28676101 Free PMC article. No abstract available.
References
-
- Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light RB, Poole L, Allred R, Constant J, Pennington J, Porter S. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;17:929–933. - PubMed
-
- Ranieri VM, Thompson BT, Barie PS, Dhainaut J, Douglas IS, Finfer S, Gãrdlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;17:2055–2064. doi: 10.1056/NEJMoa1202290. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

