Melanocyte-specific CD8+ T cells are associated with epidermal depigmentation in a novel mouse model of vitiligo
- PMID: 23711243
- PMCID: PMC3784211
- DOI: 10.1111/cei.12146
Melanocyte-specific CD8+ T cells are associated with epidermal depigmentation in a novel mouse model of vitiligo
Abstract
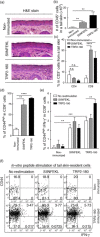
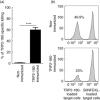
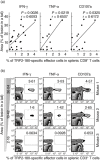
In the present study, we established a novel murine model of vitiligo by sequential prime/boost immunizations into the hind footpad and tail dermis with tyrosinase-related protein 2 (TRP2)-180 (SVYDFFVWL) peptide, lipopolysaccharides and cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides. Immunized mice developed epidermal depigmentation in the tail skin without hair depigmentation, thereby differentiating this approach from established models of vitiligo. Following intradermal tail immunization, activated CD8(+) interferon (IFN)-γ(+) T cells were recruited locally to the tail skin. In-vivo cytotoxicity assays demonstrated specific lysis of TRP2-180-presenting cells in immunized mice. Furthermore, the extent of skin depigmentation correlated with the frequency of TRP2-180-specific splenic CD8(+) T cells, as determined by IFN-γ and tumour necrosis factor (TNF)-α production, and cytotoxic degranulation evidenced by CD107a staining. These findings suggest a correlation between the presence of TRP2-180-specific CD8(+) effector T cells and the development of depigmented skin lesions in our vitiligo model. This new model of vitiligo, characterized by skin depigmentation without hair depigmentation, is more similar to human disease than previous murine models. Therefore, this model is well suited to future studies on the pathogenesis of vitiligo and the development of novel therapeutics for vitiligo.
Keywords: autoreactive CD8+ T cell; epidermal depigmentation; mouse model; vitiligo.
© 2013 British Society for Immunology.
Figures


References
-
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419–431. - PubMed
-
- Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview: part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–491. - PubMed
-
- Palermo B, Campanelli R, Garbelli S, et al. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol. 2001;117:326–332. - PubMed
-
- Le Gal FA, Avril MF, Bosq J, et al. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117:1464–1470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

