Inhibition of x-box binding protein 1 reduces tunicamycin-induced apoptosis in aged murine macrophages
- PMID: 23711292
- PMCID: PMC3773058
- DOI: 10.1111/acel.12105
Inhibition of x-box binding protein 1 reduces tunicamycin-induced apoptosis in aged murine macrophages
Abstract
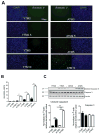
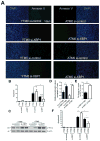
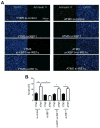
Endoplasmic reticulum (ER) stress is induced by the accumulation of unfolded and misfolded proteins in the ER. Although apoptosis induced by ER stress has been implicated in several aging-associated diseases, such as atherosclerosis, it is unclear how aging modifies ER stress response in macrophages. To decipher this relationship, we assessed apoptosis in macrophages isolated from young (1.5-2 months) and aged (16-18 months) mice and exposed the cells to the ER stress inducer tunicamycin. We found that aged macrophages exhibited more apoptosis than young macrophages, which was accompanied by reduced activation of phosphorylated inositol-requiring enzyme-1 (p-IRE1α), one of the three key ER stress signal transducers. Reduced gene expression of x-box binding protein 1 (XBP1), a downstream effector of IRE1α, enhanced p-IRE1α levels and reduced apoptosis in aged, but not young macrophages treated with tunicamycin. These findings delineate a novel, age-dependent interaction by which macrophages undergo apoptosis upon ER stress, and suggest an important protective role of IRE1α in aging-associated ER stress-induced apoptosis. This novel pathway may not only be important in our understanding of longevity, but may also have important implications for pathogenesis and potential treatment of aging-associated diseases in general.
Keywords: aging; apoptosis; endoplasmic reticulum stress; macrophages.
© 2013 The Anatomical Society and John Wiley & Sons Ltd.
Figures


Similar articles
-
Endoplasmic reticulum stress in retinal vascular degeneration: protective role of resveratrol.Invest Ophthalmol Vis Sci. 2012 May 31;53(6):3241-9. doi: 10.1167/iovs.11-8406. Invest Ophthalmol Vis Sci. 2012. PMID: 22491413
-
Up-regulation of GRP78 and antiapoptotic signaling in murine peritoneal macrophages exposed to insulin.J Leukoc Biol. 2005 Jul;78(1):187-94. doi: 10.1189/jlb.1104685. Epub 2005 Apr 21. J Leukoc Biol. 2005. PMID: 15845644 Free PMC article.
-
HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction.PLoS Biol. 2010 Jul 6;8(7):e1000410. doi: 10.1371/journal.pbio.1000410. PLoS Biol. 2010. PMID: 20625543 Free PMC article.
-
Targeting the IRE1α-XBP1 branch of the unfolded protein response in human diseases.Semin Cancer Biol. 2015 Aug;33:48-56. doi: 10.1016/j.semcancer.2015.04.010. Epub 2015 May 16. Semin Cancer Biol. 2015. PMID: 25986851 Free PMC article. Review.
-
The Stress of Lung Aging: Endoplasmic Reticulum and Senescence Tête-à-Tête.Physiology (Bethesda). 2021 May 1;36(3):150-159. doi: 10.1152/physiol.00039.2020. Physiology (Bethesda). 2021. PMID: 33904785 Review.
Cited by
-
Involvement of the IRE1α-XBP1 pathway and XBP1s-dependent transcriptional reprogramming in metabolic diseases.DNA Cell Biol. 2015 Jan;34(1):6-18. doi: 10.1089/dna.2014.2552. DNA Cell Biol. 2015. PMID: 25216212 Free PMC article. Review.
-
Schlafen2 mutation unravels a role for chronic ER stress in the loss of T cell quiescence.Oncotarget. 2016 Jun 28;7(26):39396-39407. doi: 10.18632/oncotarget.9818. Oncotarget. 2016. PMID: 27276683 Free PMC article.
-
Endoplasmic Reticulum Stress and miRNA Impairment in Aging and Age-Related Diseases.Front Aging. 2022 Jan 20;2:790702. doi: 10.3389/fragi.2021.790702. eCollection 2021. Front Aging. 2022. PMID: 35822008 Free PMC article. Review.
-
Endoplasmic reticulum proteostasis impairment in aging.Aging Cell. 2017 Aug;16(4):615-623. doi: 10.1111/acel.12599. Epub 2017 Apr 23. Aging Cell. 2017. PMID: 28436203 Free PMC article. Review.
-
Role of Endoplasmic Reticulum Stress in Atherosclerosis and Its Potential as a Therapeutic Target.Oxid Med Cell Longev. 2020 Sep 9;2020:9270107. doi: 10.1155/2020/9270107. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32963706 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

