Oscillations and the basal ganglia: motor control and beyond
- PMID: 23711535
- PMCID: PMC4813758
- DOI: 10.1016/j.neuroimage.2013.05.084
Oscillations and the basal ganglia: motor control and beyond
Abstract
Oscillations form a ubiquitous feature of the central nervous system. Evidence is accruing from cortical and sub-cortical recordings that these rhythms may be functionally important, although the precise details of their roles remain unclear. The basal ganglia share this predilection for rhythmic activity which, as we see in Parkinson's disease, becomes further enhanced in the dopamine depleted state. While certain cortical rhythms appear to penetrate the basal ganglia, others are transformed or blocked. Here, we discuss the functional association of oscillations in the basal ganglia and their relationship with cortical activity. We further explore the neural underpinnings of such oscillatory activity, including the important balance to be struck between facilitating information transmission and limiting information coding capacity. Finally, we introduce the notion that synchronised oscillatory activity can be broadly categorised as immutability promoting rhythms that reinforce incumbent processes, and mutability promoting rhythms that favour novel processing.
Keywords: Basal ganglia; Cross-frequency; Deep brain stimulation; Immutable; Information theory; Parkinson's disease.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

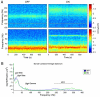

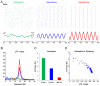
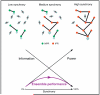
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alegre M, Alonso-Frech F, Rodríguez-Oroz MC, Guridi J, Zamarbide I, Valencia M, Manrique M, Obeso JA, Artieda J. Movement-related changes in oscillatory activity in the human subthalamic nucleus: ipsilateral vs. contralateral movements. Eur. J. Neurosci. 2005;22:2315–2324. - PubMed
-
- Alegre M, Lopez-Azcarate J, Obeso I, Wilkinson L, Rodriguez-Oroz MC, Valencia M, Garcia-Garcia D, Guridi J, Artieda J, Jahanshahi M, Obeso JA. The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson’s disease. Exp. Neurol. 2013;239:1–12. - PubMed
-
- Alonso-Frech F. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain. 2006;129:1748–1757. - PubMed
-
- Androulidakis AG, Doyle LMF, Yarrow K, Litvak V, Gilbertson TP, Brown P. Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. Eur. J. Neurosci. 2007;25:3758–3765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

