Use of allosteric targets in the discovery of safer drugs
- PMID: 23711993
- PMCID: PMC5586781
- DOI: 10.1159/000350417
Use of allosteric targets in the discovery of safer drugs
Abstract
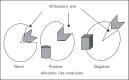
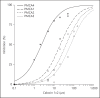
The need for drugs with fewer side effects cannot be overemphasized. Today, most drugs modify the actions of enzymes, receptors, transporters and other molecules by directly binding to their active (orthosteric) sites. However, orthosteric site configuration is similar in several proteins performing related functions and this leads to a lower specificity of a drug for the desired protein. Consequently, such drugs may have adverse side effects. A new basis of drug discovery is emerging based on the binding of the drug molecules to sites away (allosteric) from the orthosteric sites. It is possible to find allosteric sites which are unique and hence more specific as targets for drug discovery. Of many available examples, two are highlighted here. The first is caloxins - a new class of highly specific inhibitors of plasma membrane Ca²⁺ pumps. The second concerns the modulation of receptors for the neurotransmitter acetylcholine, which binds to 12 types of receptors. Exploitation of allosteric sites has led to the discovery of drugs which can selectively modulate the activation of only 1 (M1 muscarinic) out of the 12 different types of acetylcholine receptors. These drugs are being tested for schizophrenia treatment. It is anticipated that the drug discovery exploiting allosteric sites will lead to more effective therapeutic agents with fewer side effects.
Copyright © 2013 S. Karger AG, Basel.
Figures
References
-
- Patwardhan B. Ethnopharmacology and drug discovery. J Ethnopharmacol. 2005;100:50–52. - PubMed
-
- Pina AS, Hussain A, Roque AC. An historical overview of drug discovery. Methods Mol Biol. 2009;572:3–12. - PubMed
-
- Warren JV. William Withering revisited: 200 years of the foxglove. Am J Cardiol. 1986;58:189–190. - PubMed
-
- Mavromoustakos T, Durdagi S, Koukoulitsa C, et al. Strategies in the rational drug design. Curr Med Chem. 2011;18:2517–2530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

