The Neurospora photoreceptor VIVID exerts negative and positive control on light sensing to achieve adaptation
- PMID: 23712010
- PMCID: PMC4039372
- DOI: 10.1038/msb.2013.24
The Neurospora photoreceptor VIVID exerts negative and positive control on light sensing to achieve adaptation
Abstract
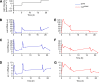
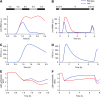
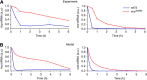
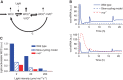
The light response in Neurospora is mediated by the photoreceptor and circadian transcription factor White Collar Complex (WCC). The expression rate of the WCC target genes adapts in daylight and remains refractory to moonlight, despite the extraordinary light sensitivity of the WCC. To explain this photoadaptation, feedback inhibition by the WCC interaction partner VIVID (VVD) has been invoked. Here we show through data-driven mathematical modeling that VVD allows Neurospora to detect relative changes in light intensity. To achieve this behavior, VVD acts as an inhibitor of WCC-driven gene expression and, at the same time, as a positive regulator that maintains the responsiveness of the photosystem. Our data indicate that this paradoxical function is realized by a futile cycle that involves the light-induced sequestration of active WCC by VVD and the replenishment of the activatable WCC pool through the decay of the photoactivated state. Our quantitative study uncovers a novel network motif for achieving sensory adaptation and defines a core input module of the circadian clock in Neurospora.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Arpaia G, Cerri F, Baima S, Macino G (1999) Involvement of protein kinase C in the response of Neurospora crassa to blue light. Mol Gen Genet 262: 314–322 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

