The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium
- PMID: 23712352
- PMCID: PMC4018742
- DOI: 10.1038/nrmicro3032
The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium
Abstract
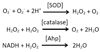
Oxic environments are hazardous. Molecular oxygen adventitiously abstracts electrons from many redox enzymes, continuously forming intracellular superoxide and hydrogen peroxide. These species can destroy the activities of metalloenzymes and the integrity of DNA, forcing organisms to protect themselves with scavenging enzymes and repair systems. Nevertheless, elevated levels of oxidants quickly poison bacteria, and both microbial competitors and hostile eukaryotic hosts exploit this vulnerability by assaulting these bacteria with peroxides or superoxide-forming antibiotics. In response, bacteria activate elegant adaptive strategies. In this Review, I summarize our current knowledge of oxidative stress in Escherichia coli, the model organism for which our understanding of damage and defence is most well developed.
Figures





References
-
-
Anbar AD. Elements and evolution. Science. 2008;322:1481–1483. A clear overview of how metal availability has changed over geological timescales.
-
-
- Ligeza A, Tikhonov AN, Hyde JS, Subczynski WK. Oxygen permeability of thylakoid memranes: electron paramagnetic resonance spin labeling study. Biochim. Biophys. Acta. 1998;1365:453–463. - PubMed
-
- Boehme DE, Vincent K, Brown OR. Oxygen and toxicity: inhibition of amino acid biosynthesis. Nature. 1976;262:418–420. - PubMed
-
-
Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and X-irradiation: a mechanism in common. Science. 1954;119:623–626. This work initiated the notion that radicals lie at the root of oxygen toxicity.
-
-
-
Loew O. A new enzyme of general occurrence in organismis. Science. 1900;11:701–702. This paper reports the discovery of catalase, the first known scavenging enzyme.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

