Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies
- PMID: 23712429
- PMCID: PMC3674693
- DOI: 10.1084/jem.20130221
Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies
Abstract
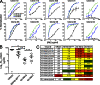
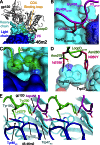
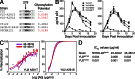
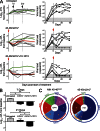
Recently identified broadly neutralizing antibodies (bNAbs) that potently neutralize most HIV-1 strains are key to potential antibody-based therapeutic approaches to combat HIV/AIDS in the absence of an effective vaccine. Increasing bNAb potencies and resistance to common routes of HIV-1 escape through mutation would facilitate their use as therapeutics. We previously used structure-based design to create the bNAb NIH45-46(G54W), which exhibits superior potency and/or breadth compared with other bNAbs. We report new, more effective NIH45-46(G54W) variants designed using analyses of the NIH45-46-gp120 complex structure and sequences of NIH45-46(G54W)-resistant HIV-1 strains. One variant, 45-46m2, neutralizes 96% of HIV-1 strains in a cross-clade panel and viruses isolated from an HIV-infected individual that are resistant to all other known bNAbs, making it the single most broad and potent anti-HIV-1 antibody to date. A description of its mechanism is presented based on a 45-46m2-gp120 crystal structure. A second variant, 45-46m7, designed to thwart HIV-1 resistance to NIH45-46(G54W) arising from mutations in a gp120 consensus sequence, targets a common route of HIV-1 escape. In combination, 45-46m2 and 45-46m7 reduce the possible routes for the evolution of fit viral escape mutants in HIV-1YU-2-infected humanized mice, with viremic control exhibited when a third antibody, 10-1074, was added to the combination.
Figures






References
-
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W., et al. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221 10.1107/S0907444909052925 - DOI - PMC - PubMed
-
- Baba T.W., Liska V., Hofmann-Lehmann R., Vlasak J., Xu W., Ayehunie S., Cavacini L.A., Posner M.R., Katinger H., Stiegler G., et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 10.1038/72309 - DOI - PubMed
-
- Baenziger S., Tussiwand R., Schlaepfer E., Mazzucchelli L., Heikenwalder M., Kurrer M.O., Behnke S., Frey J., Oxenius A., Joller H., et al. 2006. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/-gamma c-/- mice. Proc. Natl. Acad. Sci. USA. 103:15951–15956 10.1073/pnas.0604493103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

