A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta
- PMID: 23712433
- PMCID: PMC3674703
- DOI: 10.1084/jem.20122753
A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta
Abstract
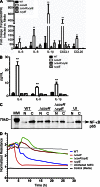
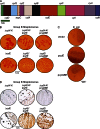
Microbial infection of the amniotic fluid is a significant cause of fetal injury, preterm birth, and newborn infections. Group B Streptococcus (GBS) is an important human bacterial pathogen associated with preterm birth, fetal injury, and neonatal mortality. Although GBS has been isolated from amniotic fluid of women in preterm labor, mechanisms of in utero infection remain unknown. Previous studies indicated that GBS are unable to invade human amniotic epithelial cells (hAECs), which represent the last barrier to the amniotic cavity and fetus. We show that GBS invades hAECs and strains lacking the hemolysin repressor CovR/S accelerate amniotic barrier failure and penetrate chorioamniotic membranes in a hemolysin-dependent manner. Clinical GBS isolates obtained from women in preterm labor are hyperhemolytic and some are associated with covR/S mutations. We demonstrate for the first time that hemolytic and cytolytic activity of GBS is due to the ornithine rhamnolipid pigment and not due to a pore-forming protein toxin. Our studies emphasize the importance of the hemolytic GBS pigment in ascending infection and fetal injury.
Figures







References
-
- Ala-Kokko T.I., Myllynen P., Vahakangas K. 2000. Ex vivo perfusion of the human placental cotyledon: implications for anesthetic pharmacology. Int. J. Obstet. Anesth. 9:26–38 10.1054/ijoa.1999.0312 - DOI
-
- Bebien M., Hensler M.E., Davanture S., Hsu L.C., Karin M., Park J.M., Alexopoulou L., Liu G.Y., Nizet V., Lawrence T. 2012. The pore-forming toxin β hemolysin/cytolysin triggers p38 MAPK-dependent IL-10 production in macrophages and inhibits innate immunity. PLoS Pathog. 8:e1002812 10.1371/journal.ppat.1002812 - DOI - PMC - PubMed
-
- Behrman R.E., Butler A.S., 2007. Preterm Birth: Causes, Consequences and Prevention. The National Academies Press, Washington, D.C. 792 pp - PubMed
-
- Bobitt J.R., Ledger W.J. 1977. Unrecognized amnionitis and prematurity: a preliminary report. J. Reprod. Med. 19:8–12 - PubMed
-
- Bourne G.L. 1960. The microscopic anatomy of the human amnion and chorion. Am. J. Obstet. Gynecol. 79:1070–1073 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007509/AI/NIAID NIH HHS/United States
- 5 T32 HD007233-29/HD/NICHD NIH HHS/United States
- K08AI067910/AI/NIAID NIH HHS/United States
- R01AI31871/AI/NIAID NIH HHS/United States
- R01 AI031871/AI/NIAID NIH HHS/United States
- K08 AI067910/AI/NIAID NIH HHS/United States
- T32 HD007233/HD/NICHD NIH HHS/United States
- R01AI070749/AI/NIAID NIH HHS/United States
- T32 AI07509/AI/NIAID NIH HHS/United States
- R01 AI070749/AI/NIAID NIH HHS/United States
- R01AI100989/AI/NIAID NIH HHS/United States
- R01 AI100989/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

