Effects of visceral inputs on the processing of labyrinthine signals by the inferior and caudal medial vestibular nuclei: ramifications for the production of motion sickness
- PMID: 23712685
- PMCID: PMC3706452
- DOI: 10.1007/s00221-013-3568-3
Effects of visceral inputs on the processing of labyrinthine signals by the inferior and caudal medial vestibular nuclei: ramifications for the production of motion sickness
Abstract
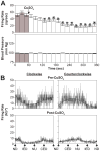
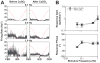
Neurons located in the caudal aspect of the vestibular nucleus complex have been shown to receive visceral inputs and project to brainstem regions that participate in generating emesis, such as nucleus tractus solitarius and the "vomiting region" in the lateral tegmental field (LTF). Consequently, it has been hypothesized that neurons in the caudal vestibular nuclei participate in triggering motion sickness and that visceral inputs to the vestibular nucleus complex can affect motion sickness susceptibility. To obtain supporting evidence for this hypothesis, we determined the effects of intragastric infusion of copper sulfate (CuSO4) on responses of neurons in the inferior and caudal medial vestibular nuclei to rotations in vertical planes. CuSO4 readily elicits nausea and emesis by activating gastrointestinal (GI) afferents. Infusion of CuSO4 produced a >30 % change in spontaneous firing rate of approximately one-third of neurons in the caudal aspect of the vestibular nucleus complex. These changes in firing rate developed over several minutes, presumably in tandem with the emetic response. The gains of responses to vertical vestibular stimulation of a larger fraction (approximately two-thirds) of caudal vestibular nucleus neurons were altered over 30 % by administration of CuSO4. The response gains of some units went up, and others went down, and there was no significant relationship with concurrent spontaneous firing rate change. These findings support the notion that the effects of visceral inputs on motion sickness susceptibility are mediated in part through the caudal vestibular nuclei. However, our previous studies showed that infusion of CuSO4 produced larger changes in response to vestibular stimulation of LTF neurons, as well as parabrachial nucleus neurons that are believed to participate in generating nausea. Thus, integrative effects of GI inputs on the processing of labyrinthine inputs must occur at brain sites that participate in eliciting motion sickness in addition to the caudal vestibular nuclei. It seems likely that the occurrence of motion sickness requires converging inputs to brain areas that generate nausea and vomiting from a variety of regions that process vestibular signals.
Figures



References
-
- Anderson JH, Blanks RHI, Precht W. Response characteristics of semicircular canal and otolith systems in the cat. I. Dynamic responses of primary vestibular fibers. Exp Brain Res. 1978;32:491–507. - PubMed
-
- Andrezik JA, Dormer KJ, Foreman RD, Person RJ. Fastigial nucleus projections to the brain stem in beagles: pathways for autonomic regulation. Neurosci. 1984;11:497–507. - PubMed
-
- Angaut P, Brodal A. The projection of the “vestibulocerebellum” onto the vestibular nuclei in the cat. Arch Ital Biol. 1967;105:441–479. - PubMed
-
- Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett. 2000;286:123–126. - PubMed
-
- Baker J, Goldberg J, Hermann G, Peterson B. Spatial and temporal response properties of secondary neurons that receive convergent input in vestibular nuclei of alert cats. Brain Res. 1984;294:138–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

