Stabilization of Kv4 protein by the accessory K(+) channel interacting protein 2 (KChIP2) subunit is required for the generation of native myocardial fast transient outward K(+) currents
- PMID: 23713033
- PMCID: PMC3779109
- DOI: 10.1113/jphysiol.2013.255836
Stabilization of Kv4 protein by the accessory K(+) channel interacting protein 2 (KChIP2) subunit is required for the generation of native myocardial fast transient outward K(+) currents
Abstract
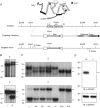
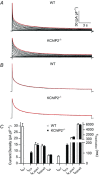
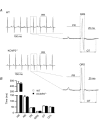
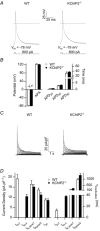
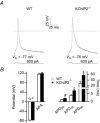
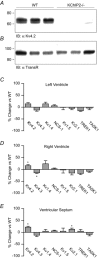
The fast transient outward K(+) current (Ito,f) underlies the early phase of myocardial action potential repolarization, contributing importantly to the coordinated propagation of activity in the heart and to the generation of normal cardiac rhythms. Native Ito,f channels reflect the tetrameric assembly of Kv4 pore-forming (α) subunits, and previous studies suggest roles for accessory and regulatory proteins in controlling the cell surface expression and the biophysical properties of Kv4-encoded Ito,f channels. Here, we demonstrate that the targeted deletion of the cytosolic accessory subunit, K(+) channel interacting protein 2 (KChIP2), results in the complete loss of the Kv4.2 protein, the α subunit critical for the generation of mouse ventricular Ito,f. Expression of the Kcnd2 (Kv4.2) transcript in KChIP2(-/-) ventricles, however, is unaffected. The loss of the Kv4.2 protein results in the elimination of Ito,f in KChIP2(-/-) ventricular myocytes. In parallel with the elimination of Ito,f, the slow transient outward K(+) current (Ito,s) is upregulated and voltage-gated Ca(2+) currents (ICa,L) are decreased. In addition, surface electrocardiograms and ventricular action potential waveforms in KChIP2(-/-) and wild-type mice are not significantly different, suggesting that the upregulation of Ito,s and the reduction in ICa,L compensate for the loss of Ito,f. Additional experiments revealed that Ito,f is not 'rescued' by adenovirus-mediated expression of KChIP2 in KChIP2(-/-) myocytes, although ICa,L densities are increased. Taken together, these results demonstrate that association with KChIP2 early in the biosynthetic pathway and KChIP2-mediated stabilization of Kv4 protein are critical determinants of native cardiac Ito,f channel expression.
Figures


Similar articles
-
Kv4.3-Encoded Fast Transient Outward Current Is Presented in Kv4.2 Knockout Mouse Cardiomyocytes.PLoS One. 2015 Jul 21;10(7):e0133274. doi: 10.1371/journal.pone.0133274. eCollection 2015. PLoS One. 2015. PMID: 26196737 Free PMC article.
-
Prolonged leptin treatment increases transient outward K⁺ current via upregulation of Kv4.2 and Kv4.3 channel subunits in adult rat ventricular myocytes.Pflugers Arch. 2014 May;466(5):903-14. doi: 10.1007/s00424-013-1348-3. Epub 2013 Sep 18. Pflugers Arch. 2014. PMID: 24046152
-
Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2.Am J Physiol Heart Circ Physiol. 2014 Apr 1;306(7):H1054-65. doi: 10.1152/ajpheart.00414.2013. Epub 2014 Jan 31. Am J Physiol Heart Circ Physiol. 2014. PMID: 24486512
-
Pharmacological Approaches for the Modulation of the Potassium Channel KV4.x and KChIPs.Int J Mol Sci. 2021 Jan 31;22(3):1419. doi: 10.3390/ijms22031419. Int J Mol Sci. 2021. PMID: 33572566 Free PMC article. Review.
-
KV Channel-Interacting Proteins in the Neurological and Cardiovascular Systems: An Updated Review.Cells. 2023 Jul 20;12(14):1894. doi: 10.3390/cells12141894. Cells. 2023. PMID: 37508558 Free PMC article. Review.
Cited by
-
New insights into the complex effects of KChIP2 on calcium transients.Am J Physiol Heart Circ Physiol. 2015 Aug 15;309(4):H553-4. doi: 10.1152/ajpheart.00511.2015. Epub 2015 Jul 10. Am J Physiol Heart Circ Physiol. 2015. PMID: 26163446 Free PMC article. No abstract available.
-
Arrhythmia: 100 years on from George Ralph Mines.J Physiol. 2013 Sep 1;591(17):4065-6. doi: 10.1113/jphysiol.2013.262386. J Physiol. 2013. PMID: 24742770 Free PMC article. No abstract available.
-
KChIP2 is a core transcriptional regulator of cardiac excitability.Elife. 2017 Mar 6;6:e17304. doi: 10.7554/eLife.17304. Elife. 2017. PMID: 28263709 Free PMC article.
-
Early remodeling of repolarizing K+ currents in the αMHC403/+ mouse model of familial hypertrophic cardiomyopathy.J Mol Cell Cardiol. 2017 Feb;103:93-101. doi: 10.1016/j.yjmcc.2017.01.006. Epub 2017 Jan 13. J Mol Cell Cardiol. 2017. PMID: 28089740 Free PMC article.
-
Potassium Channel Interacting Protein 2 (KChIP2) is not a transcriptional regulator of cardiac electrical remodeling.Sci Rep. 2016 Jun 28;6:28760. doi: 10.1038/srep28760. Sci Rep. 2016. PMID: 27349185 Free PMC article.
References
-
- Aimond F, Kwak SP, Rhodes KJ, Nerbonne JM. Accessory Kvβ1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes. Circ Res. 2005;96:451–458. - PubMed
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Antzelevitch C, Sicouri S, Litovsky S, Lukas A, Krishnan S, Di Diego J, Gintant G, Liu D. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–1449. - PubMed
-
- Bähring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. J Biol Chem. 2001;276:23888–23894. - PubMed
-
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Circ Res. 1998;83:560–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

