Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial
- PMID: 23713082
- PMCID: PMC3743070
- DOI: 10.1093/eurheartj/eht187
Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial
Abstract
Aims: Mineralocorticoid receptor antagonists (MRAs) improve outcomes in patients with heart failure and reduced left ventricular ejection fraction (HFrEF), but their use is limited by hyperkalaemia and/or worsening renal function (WRF). BAY 94-8862 is a highly selective and strongly potent non-steroidal MRA. We investigated its safety and tolerability in patients with HFrEF associated with mild or moderate chronic kidney disease (CKD).
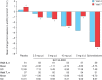
Methods and results: This randomized, controlled, phase II trial consisted of two parts. In part A, the safety and tolerability of oral BAY 94-8862 [2.5, 5, or 10 mg once daily (q.d.)] was assessed in 65 patients with HFrEF and mild CKD. In part B, BAY 94-8862 (2.5, 5, or 10 mg q.d., or 5 mg twice daily) was compared with placebo and open-label spironolactone (25 or 50 mg/day) in 392 patients with HFrEF and moderate CKD. BAY 94-8862 was associated with significantly smaller mean increases in serum potassium concentration than spironolactone (0.04-0.30 and 0.45 mmol/L, respectively, P < 0.0001-0.0107) and lower incidences of hyperkalaemia (5.3 and 12.7%, respectively, P = 0.048) and WRF. BAY 94-8862 decreased the levels of B-type natriuretic peptide (BNP), amino-terminal proBNP, and albuminuria at least as much as spironolactone. Adverse events related to BAY 94-8862 were infrequent and mostly mild.
Conclusion: In patients with HFrEF and moderate CKD, BAY 94-8862 5-10 mg/day was at least as effective as spironolactone 25 or 50 mg/day in decreasing biomarkers of haemodynamic stress, but it was associated with lower incidences of hyperkalaemia and WRF.
Keywords: Aldosterone; Antagonist; Chronic kidney disease; Heart failure; Mineralocorticoid receptor.
Figures




Comment in
-
The ARTS of third-generation mineralocorticoid receptor antagonists: achieving cardiovascular benefit with minimized renal side effects?Eur Heart J. 2013 Aug;34(31):2426-8. doi: 10.1093/eurheartj/eht235. Epub 2013 Jul 3. Eur Heart J. 2013. PMID: 23824829 No abstract available.
References
-
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi:10.1056/NEJMoa1009492. - DOI - PubMed
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi:10.1056/NEJM199909023411001. - DOI - PubMed
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi:10.1093/eurheartj/ehs104. - DOI - PubMed
-
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi:10.1016/j.jacc.2008.11.013. - DOI - PubMed
-
- Bärfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa-Perez S, Heckroth H, Nitsche A, Erguden JK, Gielen-Haertwig H, Schlemmer KH, Mittendorf J, Paulsen H, Platzek J, Kolkhof P. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7:1385–1403. doi:10.1002/cmdc.201200081. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

