LGI proteins in the nervous system
- PMID: 23713523
- PMCID: PMC3691968
- DOI: 10.1042/AN20120095
LGI proteins in the nervous system
Abstract
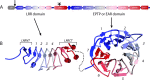
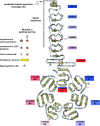
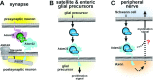
The development and function of the vertebrate nervous system depend on specific interactions between different cell types. Two examples of such interactions are synaptic transmission and myelination. LGI1-4 (leucine-rich glioma inactivated proteins) play important roles in these processes. They are secreted proteins consisting of an LRR (leucine-rich repeat) domain and a so-called epilepsy-associated or EPTP (epitempin) domain. Both domains are thought to function in protein-protein interactions. The first LGI gene to be identified, LGI1, was found at a chromosomal translocation breakpoint in a glioma cell line. It was subsequently found mutated in ADLTE (autosomal dominant lateral temporal (lobe) epilepsy) also referred to as ADPEAF (autosomal dominant partial epilepsy with auditory features). LGI1 protein appears to act at synapses and antibodies against LGI1 may cause the autoimmune disorder limbic encephalitis. A similar function in synaptic remodelling has been suggested for LGI2, which is mutated in canine Benign Familial Juvenile Epilepsy. LGI4 is required for proliferation of glia in the peripheral nervous system and binds to a neuronal receptor, ADAM22, to foster ensheathment and myelination of axons by Schwann cells. Thus, LGI proteins play crucial roles in nervous system development and function and their study is highly important, both to understand their biological functions and for their therapeutic potential. Here, we review our current knowledge about this important family of proteins, and the progress made towards understanding their functions.
Figures
References
-
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. - PubMed
-
- Baulac S, Ishida S, Mashimo T, Boillot M, Fumoto N, Kuwamura M, Ohno Y, Takizawa A, Aoto T, Ueda M, Ikeda A, LeGuern E, Takahashi R, Serikawa T. A rat model for LGI1-related epilepsies. Human molecular genetics. 2012;21:3546–3557. - PubMed
-
- Berghuis B, Brilstra EH, Lindhout D, Baulac S, de Haan GJ, van Kempen M. Hyperactive behavior in a family with autosomal dominant lateral temporal lobe epilepsy caused by a mutation in the LGI1/epitempin gene. Epilepsy & behavior: E&B. 2013;28:41–46. - PubMed
-
- Bermingham JR, Jr, Scherer SS, O’Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
