Peroxisome proliferation-activated receptor δ agonist GW0742 interacts weakly with multiple nuclear receptors, including the vitamin D receptor
- PMID: 23713684
- PMCID: PMC3724348
- DOI: 10.1021/bi400321p
Peroxisome proliferation-activated receptor δ agonist GW0742 interacts weakly with multiple nuclear receptors, including the vitamin D receptor
Abstract
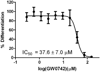
A high-throughput screening campaign was conducted to identify small molecules with the ability to inhibit the interaction between the vitamin D receptor (VDR) and steroid receptor coactivator 2. These inhibitors represent novel molecular probes for modulating gene regulation mediated by VDR. Peroxisome proliferator-activated receptor (PPAR) δ agonist GW0742 was among the identified VDR-coactivator inhibitors and has been characterized herein as a pan nuclear receptor antagonist at concentrations of > 12.1 μM. The highest antagonist activity for GW0742 was found for VDR and the androgen receptor. Surprisingly, GW0742 behaved as a PPAR agonist and antagonist, activating transcription at lower concentrations and inhibiting this effect at higher concentrations. A unique spectroscopic property of GW0742 was identified as well. In the presence of rhodamine-derived molecules, GW0742 increased the fluorescence intensity and level of fluorescence polarization at an excitation wavelength of 595 nm and an emission wavelength of 615 nm in a dose-dependent manner. The GW0742-inhibited NR-coactivator binding resulted in a reduced level of expression of five different NR target genes in LNCaP cells in the presence of agonist. Especially VDR target genes CYP24A1, IGFBP-3, and TRPV6 were negatively regulated by GW0742. GW0742 is the first VDR ligand inhibitor lacking the secosteroid structure of VDR ligand antagonists. Nevertheless, the VDR-meditated downstream process of cell differentiation was antagonized by GW0742 in HL-60 cells that were pretreated with the endogenous VDR agonist 1,25-dihydroxyvitamin D3.
Figures







References
-
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. - PubMed
-
- Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear receptor structure: implications for function. Annu Rev Physiol. 2007;69:201–220. - PubMed
-
- The Binding Database. ChemAxon. 2013
-
- Ding XF, Anderson CM, Ma H, Hong H, Uht RM, Kushner PJ, Stallcup MR. Nuclear receptor-binding sites of coactivators glucocorticoid receptor interacting protein 1 (GRIP1) and steroid receptor coactivator 1 (SRC-1): multiple motifs with different binding specificities. Mol Endocrinol. 1998;12:302–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

