Are systematic reviews up-to-date at the time of publication?
- PMID: 23714302
- PMCID: PMC3674908
- DOI: 10.1186/2046-4053-2-36
Are systematic reviews up-to-date at the time of publication?
Abstract
Background: Systematic reviews provide a synthesis of evidence for practitioners, for clinical practice guideline developers, and for those designing and justifying primary research. Having an up-to-date and comprehensive review is therefore important. Our main objective was to determine the recency of systematic reviews at the time of their publication, as measured by the time from last search date to publication. We also wanted to study the time from search date to acceptance, and from acceptance to publication, and measure the proportion of systematic reviews with recorded information on search dates and information sources in the abstract and full text of the review.
Methods: A descriptive analysis of published systematic reviews indexed in Medline in 2009, 2010 and 2011 by three reviewers, independently extracting data.
Results: Of the 300 systematic reviews included, 271 (90%) provided the date of search in the full-text article, but only 141 (47%) stated this in the abstract. The median (standard error; minimum to maximum) survival time from last search to acceptance was 5.1 (0.58; 0 to 43.8) months (95% confidence interval = 3.9 to 6.2) and from last search to first publication time was 8.0 (0.35; 0 to 46.7) months (95% confidence interval = 7.3 to 8.7), respectively. Of the 300 reviews, 295 (98%) stated which databases had been searched, but only 181 (60%) stated the databases in the abstract. Most researchers searched three (35%) or four (21%) databases. The top-three most used databases were MEDLINE (79%), Cochrane library (76%), and EMBASE (64%).
Conclusions: Being able to identify comprehensive, up-to-date reviews is important to clinicians, guideline groups, and those designing clinical trials. This study demonstrates that some reviews have a considerable delay between search and publication, but only 47% of systematic review abstracts stated the last search date and 60% stated the databases that had been searched. Improvements in the quality of abstracts of systematic reviews and ways to shorten the review and revision processes to make review publication more rapid are needed.
Figures


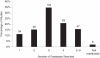
References
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

