Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer
- PMID: 23714732
- PMCID: PMC3778101
- DOI: 10.1158/1078-0432.CCR-13-0231
Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer
Abstract
Purpose: To assess the efficacy of a single infusion of radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 (lutetium-177; (177)Lu) by prostate-specific antigen (PSA) decline, measurable disease response, and survival.
Experimental design: In this dual-center phase II study, two cohorts with progressive metastatic castration-resistant prostate cancer received one dose of (177)Lu-J591 (15 patients at 65 mCi/m(2), 17 at 70 mCi/m(2)) with radionuclide imaging. Expansion cohort (n = 15) received 70 mCi/m(2) to verify response rate and examine biomarkers.
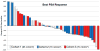
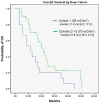
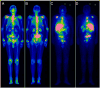
Results: Forty-seven patients who progressed after hormonal therapies (55.3% also received prior chemotherapy) received (177)Lu-J591. A total of 10.6% experienced ≥50% decline in PSA, 36.2% experienced ≥30% decline, and 59.6% experienced any PSA decline following their single treatment. One of 12 with measurable disease experienced a partial radiographic response (8 with stable disease). Sites of prostate cancer metastases were targeted in 44 of 47 (93.6%) as determined by planar imaging. All experienced reversible hematologic toxicity, with grade 4 thrombocytopenia occurring in 46.8% (29.8% received platelet transfusions) without significant hemorrhage. A total of 25.5% experienced grade 4 neutropenia, with one episode of febrile neutropenia. The phase I maximum tolerated dose (70 mCi/m(2)) resulted in more 30% PSA declines (46.9% vs. 13.3%, P = 0.048) and longer survival (21.8 vs. 11.9 months, P = 0.03), but also more grade 4 hematologic toxicity and platelet transfusions. No serious nonhematologic toxicity occurred. Those with poor PSMA imaging were less likely to respond.
Conclusion: A single dose of (177)Lu-J591 was well tolerated with reversible myelosuppression. Accurate tumor targeting and PSA responses were seen with evidence of dose response. Imaging biomarkers seem promising.
©2013 AACR.
Conflict of interest statement
Figures
References
-
- Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25:805–13. - PubMed
-
- Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol. 1994;31:33–40. - PubMed
-
- Sartor O, Reid R, Hoskin P, Quick D, Ell P, Coleman R, et al. Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–5. - PubMed
-
- Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–94. - PubMed
-
- Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

