Amyloid β precursor protein as a molecular target for amyloid β--induced neuronal degeneration in Alzheimer's disease
- PMID: 23714735
- PMCID: PMC3762679
- DOI: 10.1016/j.neurobiolaging.2013.04.021
Amyloid β precursor protein as a molecular target for amyloid β--induced neuronal degeneration in Alzheimer's disease
Abstract
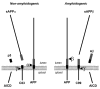
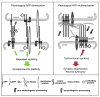
A role of amyloid β (Aβ) peptide aggregation and deposition in Alzheimer's disease (AD) pathogenesis is widely accepted. Significantly, abnormalities induced by aggregated Aβ have been linked to synaptic and neuritic degeneration, consistent with the "dying-back" pattern of degeneration that characterizes neurons affected in AD. However, molecular mechanisms underlying the toxic effect of aggregated Aβ remain elusive. In the last 2 decades, a variety of aggregated Aβ species have been identified and their toxic properties demonstrated in diverse experimental systems. Concurrently, specific Aβ assemblies have been shown to interact and misregulate a growing number of molecular effectors with diverse physiological functions. Such pleiotropic effects of aggregated Aβ posit a mayor challenge for the identification of the most cardinal Aβ effectors relevant to AD pathology. In this review, we discuss recent experimental evidence implicating amyloid β precursor protein (APP) as a molecular target for toxic Aβ assemblies. Based on a significant body of pathologic observations and experimental evidence, we propose a novel pathologic feed-forward mechanism linking Aβ aggregation to abnormalities in APP processing and function, which in turn would trigger the progressive loss of neuronal connectivity observed early in AD.
Keywords: APP; Alzheimer's disease; Aβ; Dying-back degeneration; Neuritic dystrophy; Synaptic degeneration.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statementThe authors of this work disclose that there are no conflicts of interest of any type that could inappropriately influence the work.
Figures

References
-
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. - PubMed
-
- Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–352. - PubMed
-
- Alvarez A, Alarcón R, Opazo C, Campos EO, Muñoz FJ, Calderón FH, Dajas F, Gentry MK, Doctor BP, De Mello FG, Inestrosa NC. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J Neurosci. 1998;18:3213–3223. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

