Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction
- PMID: 23714783
- PMCID: PMC3724390
- DOI: 10.1369/0022155413493912
Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction
Abstract
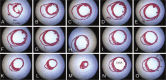
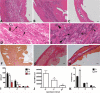
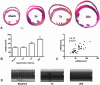
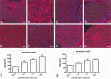
Mouse models of myocardial infarction are essential tools for the study of cardiac injury, repair, and remodeling. Our current investigation establishes a systematic approach for quantitative evaluation of the inflammatory and reparative response, cardiac function, and geometry in a mouse model of reperfused myocardial infarction. Reperfused mouse infarcts exhibited marked induction of inflammatory cytokines that peaked after 6 hr of reperfusion. In the infarcted heart, scar contraction and chamber dilation continued for at least 28 days after reperfusion; infarct maturation was associated with marked thinning of the scar, accompanied by volume loss and rapid clearance of cellular elements. Echocardiographic measurements of end-diastolic dimensions correlated well with morphometric assessment of dilative remodeling in perfusion-fixed hearts. Hemodynamic monitoring was used to quantitatively assess systolic and diastolic function; the severity of diastolic dysfunction following myocardial infarction correlated with cardiomyocyte hypertrophy and infarct collagen content. Expression of molecular mediators of inflammation and cellular infiltration needs to be investigated during the first 72 hr, whereas assessment of dilative remodeling requires measurement of geometric parameters for at least four weeks after the acute event. Rapid initiation and resolution of the inflammatory response, accelerated scar maturation, and extensive infarct volume loss are important characteristics of infarct healing in mice.
Keywords: cardiac remodeling; cardiomyocyte; cytokine; fibroblast; inflammation; myocardial infarction.
Conflict of interest statement
Figures




References
-
- Bogen DK, Rabinowitz SA, Needleman A, McMahon TA, Abelmann WH. 1980. An analysis of the mechanical disadvantage of myocardial infarction in the canine left ventricle. Circ Res. 47:728–741 - PubMed
-
- Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. 2009. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 105:973–983 - PMC - PubMed
-
- Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. 2007. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 116:2127–2138 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

