Tris(2-pyridylmethyl)amine (TPA) as a membrane-permeable chelator for interception of biological mobile zinc
- PMID: 23715510
- PMCID: PMC3730853
- DOI: 10.1039/c3mt00103b
Tris(2-pyridylmethyl)amine (TPA) as a membrane-permeable chelator for interception of biological mobile zinc
Abstract
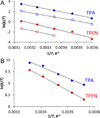
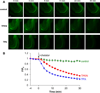
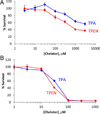
We report the characterization of tris(2-pyridylmethyl)amine (TPA) as a membrane-permeable zinc chelator for intercepting biological mobile zinc. Compared to N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), TPA chelates zinc with faster kinetics in cuvettes, live cells, and brain slices. TPA also is generally less toxic than TPEN in cell culture. Mechanistic analysis indicates that these improvements arise from both the electronic and steric properties of TPA including weaker metal-binding affinity, lower pKa, and smaller size. These results demonstrate that TPA chelation is a valuable addition to the methodologies available for investigating mobile zinc in biology.
Figures





Similar articles
-
Bis(2-quinolylmethyl)ethylenediaminediacetic acids (BQENDAs), TQEN-EDTA hybrid molecules as fluorescent zinc sensors.Dalton Trans. 2014 Jul 14;43(26):10013-22. doi: 10.1039/c4dt00261j. Epub 2014 May 23. Dalton Trans. 2014. PMID: 24853956
-
Tetrakis(2-quinolinylmethyl)ethylenediamine (TQEN) as a new fluorescent sensor for zinc.Dalton Trans. 2005 Feb 7;(3):545-50. doi: 10.1039/b411924j. Epub 2004 Dec 21. Dalton Trans. 2005. PMID: 15672199
-
Prevention of zinc neurotoxicity in vivo by N,N,N',N'-tetrakis (2-pyridylmethyl) ethylene-diamine (TPEN).Neuroreport. 1996 May 17;7(7):1301-4. doi: 10.1097/00001756-199605170-00017. Neuroreport. 1996. PMID: 8817554
-
TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro.Cell Calcium. 1998 Feb-Mar;23(2-3):151-64. doi: 10.1016/s0143-4160(98)90114-2. Cell Calcium. 1998. PMID: 9601611 Review.
-
Chelators for investigating zinc metalloneurochemistry.Curr Opin Chem Biol. 2013 Apr;17(2):129-36. doi: 10.1016/j.cbpa.2013.01.009. Epub 2013 Mar 13. Curr Opin Chem Biol. 2013. PMID: 23478014 Free PMC article. Review.
Cited by
-
Tools and techniques for illuminating the cell biology of zinc.Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118865. doi: 10.1016/j.bbamcr.2020.118865. Epub 2020 Sep 24. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 32980354 Free PMC article. Review.
-
Mobile zinc as a modulator of sensory perception.FEBS Lett. 2023 Jan;597(1):151-165. doi: 10.1002/1873-3468.14544. Epub 2022 Dec 20. FEBS Lett. 2023. PMID: 36416529 Free PMC article. Review.
-
Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate.Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):E5464-71. doi: 10.1073/pnas.1609450113. Epub 2016 Aug 25. Proc Natl Acad Sci U S A. 2016. PMID: 27562169 Free PMC article.
-
Hyperpolarized 15N-labeled, deuterated tris (2-pyridylmethyl)amine as an MRI sensor of freely available Zn2.Commun Chem. 2020;3:185. doi: 10.1038/s42004-020-00426-6. Epub 2020 Dec 9. Commun Chem. 2020. PMID: 34212118 Free PMC article.
-
Amino Acid-Derived Sensors for Specific Zn2+ Detection Using Hyperpolarized 13 C Magnetic Resonance Spectroscopy.Chemistry. 2019 Sep 12;25(51):11842-11846. doi: 10.1002/chem.201902771. Epub 2019 Aug 20. Chemistry. 2019. PMID: 31338914 Free PMC article.
References
-
- Maret W. BioMetals. 2009;22:149–157. - PubMed
-
- Vallee BL, Falchuk KH. Physiol. Rev. 1993;73:79–118. - PubMed
-
- Chang CJ, Lippard SJ. Neurodegenerative Diseases and Metal Ions. John Wiley & Sons; 2006. pp. 321–370.
-
- Sensi SL, Paoletti P, Bush AI, Sekler I. Nat. Rev. Neurosci. 2009;10:780–791. - PubMed
-
- Taylor CG. BioMetals. 2005;18:305–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

