The cotton transcription factor TCP14 functions in auxin-mediated epidermal cell differentiation and elongation
- PMID: 23715527
- PMCID: PMC3707559
- DOI: 10.1104/pp.113.215673
The cotton transcription factor TCP14 functions in auxin-mediated epidermal cell differentiation and elongation
Abstract
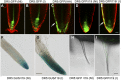
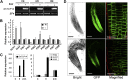
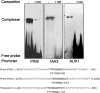
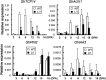
Plant-specific TEOSINTE-BRANCHED1/CYCLOIDEA/PCF (TCP) transcription factors play crucial roles in development, but their functional mechanisms remain largely unknown. Here, we characterized the cellular functions of the class I TCP transcription factor GhTCP14 from upland cotton (Gossypium hirsutum). GhTCP14 is expressed predominantly in fiber cells, especially at the initiation and elongation stages of development, and its expression increased in response to exogenous auxin. Induced heterologous overexpression of GhTCP14 in Arabidopsis (Arabidopsis thaliana) enhanced initiation and elongation of trichomes and root hairs. In addition, root gravitropism was severely affected, similar to mutant of the auxin efflux carrier PIN-FORMED2 (PIN2) gene. Examination of auxin distribution in GhTCP14-expressing Arabidopsis by observation of auxin-responsive reporters revealed substantial alterations in auxin distribution in sepal trichomes and root cortical regions. Consistent with these changes, expression of the auxin uptake carrier AUXIN1 (AUX1) was up-regulated and PIN2 expression was down-regulated in the GhTCP14-expressing plants. The association of GhTCP14 with auxin responses was also evidenced by the enhanced expression of auxin response gene IAA3, a gene in the AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) family. Electrophoretic mobility shift assays showed that GhTCP14 bound the promoters of PIN2, IAA3, and AUX1, and transactivation assays indicated that GhTCP14 had transcription activation activity. Taken together, these results demonstrate that GhTCP14 is a dual-function transcription factor able to positively or negatively regulate expression of auxin response and transporter genes, thus potentially acting as a crucial regulator in auxin-mediated differentiation and elongation of cotton fiber cells.
Figures




References
-
- Basra AS, Malik CP. (1984) Development of the cotton fibre. Int Rev Cytol 87: 65–113
-
- Benjamins R, Scheres B. (2008) Auxin: the looping star in plant development. Annu Rev Plant Biol 59: 443–465 - PubMed
-
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 - PubMed
-
- Cedroni ML, Cronn RC, Adams KL, Wilkins TA, Wendel JF. (2003) Evolution and expression of MYB genes in diploid and polyploid cotton. Plant Mol Biol 51: 313–325 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

