Affinity and activity of non-native quinones at the Q(B) site of bacterial photosynthetic reaction centers
- PMID: 23715773
- PMCID: PMC4442677
- DOI: 10.1007/s11120-013-9850-1
Affinity and activity of non-native quinones at the Q(B) site of bacterial photosynthetic reaction centers
Abstract
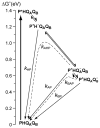
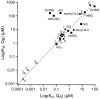
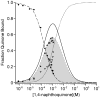
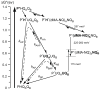
Purple, photosynthetic reaction centers from Rhodobacter sphaeroides bacteria use ubiquinone (UQ10) as both primary (Q(A)) and secondary (Q(B)) electron acceptors. Many quinones reconstitute Q(A) function, while a few will act as Q(B). Nine quinones were tested for their ability to bind and reconstitute Q(A) and Q(B) functions. Only ubiquinone (UQ) reconstitutes both functions in the same protein. The affinities of the non-native quinones for the Q(B) site were determined by a competitive inhibition assay. The affinities of benzoquinones, naphthoquinone (NQ), and 2-methyl-NQ for the Q(B) site are 7 ± 3 times weaker than that at Q(A) site. However, di-ortho-substituted NQs and anthraquinone bind tightly to the Q(A) site (K d ≤ 200 nM), and ≥1,000 times more weakly to the Q(B) site, perhaps setting a limit on the size of the site. With a low-potential electron donor, 2-methyl, 3-dimethylamino-1,4-NQ, (Me-diMeAm-NQ) at Q(A), Q(B) reduction is 260 meV, more favorable than with UQ as Q(A). Electron transfer from Me-diMeAm-NQ at the Q(A) site to NQ at the Q(B) site can be detected. In the Q(B) site, the NQ semiquinone is estimated to be ≈60-100 meV higher in energy than the UQ semiquinone, while in the Q(A) site, the semiquinone energy level is similar or lower with NQ than with UQ. Thus, the NQ semiquinone is more stable in the Q(A) than in the Q(B) site. In contrast, the native UQ semiquinone is ≈60 meV lower in energy in the Q(B) than in the Q(A) site, stabilizing forward electron transfer from Q(A) to Q(B).
Figures




References
-
- Arata H, Parson WW. Delayed fluorescence from Rhodopseudomonas sphaeroides reaction centers: Enthalpy and free energy changes accompanying electron transfer from P870 to quinones. Biochim Biophys Acta. 1981;638:201–209. - PubMed
-
- Ashnagar A, Bruce M, Dutton PL, Prince R. One- and two-electron reduction of hydroxy-1,4-naphthoquinone and hydroxy-9,10-anthraquinones. The role of internal hydrogen bonding and its bearing on the redox chemistry of the anthracycline antitumour quinones. Biochim Biophys Acta. 1984;801:351–359. - PubMed
-
- Baccarini-Melandri A, Gabellini N, Melandri BA, Hurt E, Hauska G. Structural requirements of quinone coenzymes for endogenous and dye-mediated coupled electron transport in bacterial photosynthesis. J Bioenerg Biomembr. 1980;12:95–110. - PubMed
-
- Blankenship RE, Parson WW. The involvement of iron and ubiquinone in electron transfer reactions mediated by reaction centers from photosynthetic bacteria. Biochim Biophys Acta. 1979;545:429–444. - PubMed
-
- Cogdell RJ, Brune DC, Clayton RK. Effects of extraction and replacement of ubiquinone upon the photochemical activity of reaction centres and chromatophores from Rhodopseudomonas sphaeroides. FEBS Lett. 1974;45:344–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

