Loss of SS18-SSX1 inhibits viability and induces apoptosis in synovial sarcoma
- PMID: 23716114
- PMCID: PMC3916608
- DOI: 10.1007/s11999-013-3065-9
Loss of SS18-SSX1 inhibits viability and induces apoptosis in synovial sarcoma
Abstract
Background: Most synovial sarcomas contain a chromosomal translocation t(X;18), which results in the formation of an oncoprotein SS18-SSX critical to the viability of synovial sarcoma.
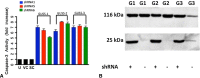
Questions/purposes: We (1) established and characterized three novel synovial sarcoma cell lines and asked (2) whether inhibition of SS18-SSX1 decreases cell viability in these cell lines; and (3) whether reduction in viability after SS18-SSX1 knockdown is caused by apoptosis. After identifying a specific posttranscriptional splice variant in our cell lines, we asked (4) whether this provides a survival benefit in synovial sarcoma.
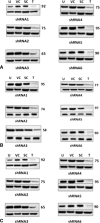
Methods: Cells lines were characterized. SS18-SSX1 knockdown was achieved using a shRNA system. Cell viability was assessed by WST-1 analysis and apoptosis examined by caspase-3 activity.
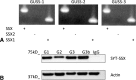
Results: We confirmed the SS18-SSX1 translocation in all cell lines and identified a consistent splicing variant. We achieved successful knockdown of SS18-SSX1 and with this saw a significant reduction in cell viability. Decreased viability was a result of increased apoptosis. Reintroduction of the exon 8 sequence into cells reduced cell viability in all cell lines.
Conclusions: We confirmed the presence of the SS18-SSX1 translocation in our cell lines and its importance in the survival of synovial sarcoma. We have also demonstrated that reduction in cell viability is related to an increase in apoptosis. In addition, we have identified a potential mediator of SS18-SSX function in exon 8.
Clinical relevance: SS18-SSX represents a tumor-specific target in synovial sarcoma. Exploitation of SS18-SSX and its protein partners will allow us to develop potent tumor-specific therapeutic agents.
Figures


References
-
- Amary MF, Berisha F, Bernardi FC, Herbert A, James M, Reis-Filho JS, Fisher C, Nicholson AG, Tirabosco R, Diss TC, Flanagan AM. Detection of SS18-SSX fusion transcripts in formalin-fixed paraffin-embedded neoplasms: analysis of conventional RT-PCR, qRT-PCR, and dual color FISH as diagnostic tools for synovial sarcoma. Mod Pathol. 2004;20:482–496. doi: 10.1038/modpathol.3800761. - DOI - PubMed
-
- Bartel F, Taylor AC, Taubert H, Harris LC. Novel mdm2 splice variants identified in pediatric rhabdomyosarcoma tumors and cell lines. Oncol Res. 2001;12:451–457. - PubMed
-
- Brodin B, Haslam K, Yang K, Bartolazzi A, Xie Y, Starborg M, Lundeberg J, Larsson O. Cloning and characterization of spliced fusion transcript variants of synovial sarcoma: SYT/SSX4, SYT/SSX4v, SYT/SSX2v. Possible regulatory role of the fusion gene product in wild type SYT expression. Gene. 2001;268:173–182. doi: 10.1016/S0378-1119(01)00412-7. - DOI - PubMed
-
- de Bruijn DR, Baats E, Zechner U, de Leeuw B, Balemans M, Olde Weghuis D, Hirning-Folz U, Geurts van Kessel AG. Isolation and characterization of the mouse homolog of SYT, a gene implicated in the development of human synovial sarcomas. Oncogene. 1996;13:643–648. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

