Steroids as γ-secretase modulators
- PMID: 23716494
- PMCID: PMC3752532
- DOI: 10.1096/fj.12-225649
Steroids as γ-secretase modulators
Abstract
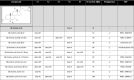
Aggregation and accumulation of Aβ42 play an initiating role in Alzheimer's disease (AD); thus, selective lowering of Aβ42 by γ-secretase modulators (GSMs) remains a promising approach to AD therapy. Based on evidence suggesting that steroids may influence Aβ production, we screened 170 steroids at 10 μM for effects on Aβ42 secreted from human APP-overexpressing Chinese hamster ovary cells. Many acidic steroids lowered Aβ42, whereas many nonacidic steroids actually raised Aβ42. Studies on the more potent compounds showed that Aβ42-lowering steroids were bonafide GSMs and Aβ42-raising steroids were inverse GSMs. The most potent steroid GSM identified was 5β-cholanic acid (EC50=5.7 μM; its endogenous analog lithocholic acid was virtually equipotent), and the most potent inverse GSM identified was 4-androsten-3-one-17β-carboxylic acid ethyl ester (EC50=6.25 μM). In addition, we found that both estrogen and progesterone are weak inverse GSMs with further complex effects on APP processing. These data suggest that certain endogenous steroids may have the potential to act as GSMs and add to the evidence that cholesterol, cholesterol metabolites, and other steroids may play a role in modulating Aβ production and thus risk for AD. They also indicate that acidic steroids might serve as potential therapeutic leads for drug optimization/development.
Keywords: Alzheimer's disease; Aβ; amyloid; cholesterol metabolites.
Figures
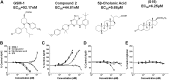



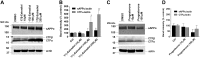
References
-
- Golde T. E., Eckman C. B., Younkin S. G. (2000) Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim. Biophys. Acta 1502, 172–187 - PubMed
-
- Teller J. K., Russo C., DeBusk L. M., Angelini G., Zaccheo D., Dagna-Bricarelli F., Scartezzini P., Bertolini S., Mann D. M., Tabaton M., Gambetti P. (1996) Presence of soluble amyloid beta-peptide precedes amyloid plaque formation in Down's syndrome. Nat. Med. 2, 93–95 - PubMed
-
- Russo C., Saido T. C., DeBusk L. M., Tabaton M., Gambetti P., Teller J. K. (1997) Heterogeneity of water-soluble amyloid β-peptide in Alzheimer's disease and Down's syndrome brains. FEBS Lett. 409, 411–416 - PubMed
-
- Wang R., Sweeney D., Gandy S., Sisodia S. (1996) The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J. Biol. Chem. 271, 31894–31902 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

