Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma
- PMID: 23716559
- PMCID: PMC3729908
- DOI: 10.3324/haematol.2013.084376
Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma
Abstract
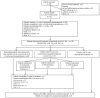
This phase II study is the first prospective evaluation of bortezomib-dexamethasone as second-line therapy for relapsed/refractory multiple myeloma. A total of 163 patients were enrolled to receive four cycles of bortezomib-dexamethasone. Patients were investigator-assessed for response at cycle 5 Day 1, then treated as follows: responding patients received another four cycles of bortezomib-dexamethasone, while patients with stable disease were subsequently randomized to sequential treatment with a further four cycles of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide. The primary end point was response to sequential therapy; however, this could not be evaluated because investigator-assessed response rates to bortezomib-dexamethasone after four cycles were high, and an insufficient number of patients were randomized to sequential treatment per protocol. Among all 163 patients, validated best confirmed response rate was 66%, including 37% complete/very good partial responses; median response duration was 9.7 months. After a median follow up of 16.9 months, median time to progression and progression-free survival were 9.5 and 8.6 months, respectively; estimated 1-year overall survival was 81%. Median glomerular filtration rate improved from baseline during treatment. Among 58 patients with baseline glomerular filtration rate below 50 mL/min, 24 had renal responses. Grade 3/4 adverse events included: thrombocytopenia (17%), anemia (10%), constipation (6%), peripheral sensory neuropathy (5%), and polyneuropathy (5%). Overall, 57% of neuropathy events improved/resolved; median time to improvement was 2.1 months. These findings suggest bortezomib-dexamethasone represents an active, feasible second-line treatment option for patients with relapsed/refractory myeloma.
Figures


References
-
- Corso A, Barbarano L, Mangiacavalli S, Spriano M, Alessandrino EP, Cafro AM, et al. Bortezomib plus dexamethasone can improve stem cell collection and overcome the need for additional chemotherapy before autologous transplant in patients with myeloma. Leuk Lymphoma. 2010;51 (2):236–42 - PubMed
-
- Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91(11):1498–505 - PubMed
-
- Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–9 - PubMed
-
- Jagannath S, Durie BG, Wolf JL, Camacho ES, Irwin D, Lutzky J, et al. Extended follow-up of a phase 2 trial of bortezomib alone and in combination with dexamethasone for the frontline treatment of multiple myeloma. Br J Haematol. 2009;146(6):619–26 - PubMed
-
- Freimann H, Calderoni A, Cornu P, Olie R. Daily practice use of Bortezomib in relapsed/refractory multiple myeloma. Safety/efficacy results of a compassionate use program in Switzerland. Swiss Med Wkly. 2007;137(21–22):317–22 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

