Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide
- PMID: 23716561
- PMCID: PMC3815166
- DOI: 10.3324/haematol.2013.083972
Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide
Abstract
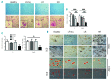
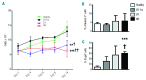
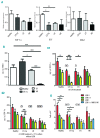
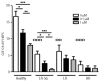
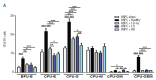
The contribution of the bone marrow microenvironment in myelodysplastic syndrome is controversial. We therefore analyzed the functional properties of primary mesenchymal stromal cells from patients with myelodysplastic syndrome in the presence or absence of lenalidomide. Compared to healthy controls, clonality and growth were reduced across all disease stages. Furthermore, differentiation defects and particular expression of adhesion and cell surface molecules (e.g. CD166, CD29, CD146) were detected. Interestingly, the levels of stromal derived factor 1-alpha in patients' cells culture supernatants were almost 2-fold lower (P<0.01) than those in controls and this was paralleled by a reduced induction of migration of CD34(+) hematopoietic cells. Co-cultures of mesenchymal stromal cells from patients with CD34(+) cells from healthy donors resulted in reduced numbers of cobblestone area-forming cells and fewer colony-forming units. Exposure of stromal cells from patients and controls to lenalidomide led to a further reduction of stromal derived factor 1-alpha secretion and cobblestone area formation, respectively. Moreover, lenalidomide pretreatment of mesenchymal stromal cells from patients with low but not high-risk myelodysplastic syndrome was able to rescue impaired erythroid and myeloid colony formation of early hematopoietic progenitors. In conclusion, our analyses support the notion that the stromal microenvironment is involved in the pathophysiology of myelodysplastic syndrome thus representing a potential target for therapeutic interventions.
Figures

References
-
- Vardiman J. The classification of MDS: from FAB to WHO and beyond. Leuk Res. 2012;36(12):1453–8 - PubMed
-
- Nimer SD. Myelodysplastic syndromes. Blood. 2008;111(10):4841–51 - PubMed
-
- List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Myelodysplastic Syndrome-003 Study Investigators Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65 - PubMed
-
- Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennet JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

