Borrelia hermsii acquisition order in superinfected ticks determines transmission efficiency
- PMID: 23716615
- PMCID: PMC3719565
- DOI: 10.1128/IAI.00542-13
Borrelia hermsii acquisition order in superinfected ticks determines transmission efficiency
Abstract
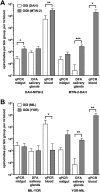
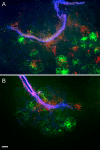
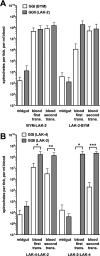
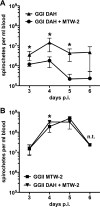
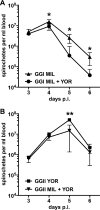
Multilocus sequence typing of Borrelia hermsii isolates reveals its divergence into two major genomic groups (GG), but no differences in transmission efficiency or host pathogenicity are associated with these genotypes. To compare GGI and GGII in the tick-host infection cycle, we first determined if spirochetes from the two groups could superinfect the tick vector Ornithodoros hermsi. We infected mice with isolates from each group and fed ticks sequentially on these mice. We then fed the infected ticks on naive mice and measured GGI and GGII spirochete densities in vector and host, using quantitative PCR of genotype-specific chromosomal DNA sequences. Sequential feedings resulted in dual tick infections, showing that GGI or GGII primary acquisition did not block superinfection by a secondary agent. On transmission to naive mice at short intervals after acquisition, ticks with primary GGI and secondary GGII spirochete infections caused mixed GGI and GGII infections in mice. However, ticks with primary GGII and secondary GGI spirochete infections caused only GGII infections with all isolate pairs examined. At longer intervals after acquisition, the exclusion of GGI by GGII spirochetes declined and cotransmission predominated. We then examined GGI and GGII spirochetemia in mice following single inoculation and coinoculation by needle and found that GGI spirochete densities were reduced on multiple days when coinoculated with GGII. These findings indicate that dual GGI-GGII spirochete infections can persist in ticks and that transmission to a vertebrate host is dependent on the order of tick acquisition and the interval between acquisition and transmission events.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

