Genetic confirmation for a central role for TNFα in the direct action of thyroid stimulating hormone on the skeleton
- PMID: 23716650
- PMCID: PMC3683712
- DOI: 10.1073/pnas.1308336110
Genetic confirmation for a central role for TNFα in the direct action of thyroid stimulating hormone on the skeleton
Erratum in
- Proc Natl Acad Sci U S A. 2013 Jul 23;110(30):12498. Baliram, Ramkumari [corrected to Baliram, Ramkumarie]
Abstract
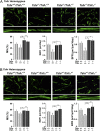
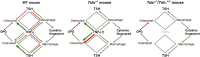
Clinical data showing correlations between low thyroid-stimulating hormone (TSH) levels and high bone turnover markers, low bone mineral density, and an increased risk of osteoporosis-related fractures are buttressed by mouse genetic and pharmacological studies identifying a direct action of TSH on the skeleton. Here we show that the skeletal actions of TSH deficiency are mediated, in part, through TNFα. Compound mouse mutants generated by genetically deleting the Tnfα gene on a Tshr(-/-) (homozygote) or Tshr(+/-) (heterozygote) background resulted in full rescue of the osteoporosis, low bone formation, and hyperresorption that accompany TSH deficiency. Studies using ex vivo bone marrow cell cultures showed that TSH inhibits and stimulates TNFα production from macrophages and osteoblasts, respectively. TNFα, in turn, stimulates osteoclastogenesis but also enhances the production in bone marrow of a variant TSHβ. This locally produced TSH suppresses osteoclast formation in a negative feedback loop. We speculate that TNFα elevations due to low TSH signaling in human hyperthyroidism contribute to the bone loss that has traditionally been attributed solely to high thyroid hormone levels.
Keywords: bone density; bone metabolism; thyroid disease.
Conflict of interest statement
Conflict of interest statement: M.Z. is a named inventor of a pending patent application related to osteoclastic bone resorption filed by the Mount Sinai School of Medicine.
Figures



References
-
- Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. - PubMed
-
- Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: The Tromsø study. Thyroid. 2008;18(11):1147–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

