ZNF408 is mutated in familial exudative vitreoretinopathy and is crucial for the development of zebrafish retinal vasculature
- PMID: 23716654
- PMCID: PMC3683717
- DOI: 10.1073/pnas.1220864110
ZNF408 is mutated in familial exudative vitreoretinopathy and is crucial for the development of zebrafish retinal vasculature
Abstract
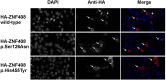
Familial exudative vitreoretinopathy (FEVR) is a genetically heterogeneous disorder characterized by abnormal vascularization of the peripheral retina, which can result in retinal detachment and severe visual impairment. In a large Dutch FEVR family, we performed linkage analysis, exome sequencing, and segregation analysis of DNA variants. We identified putative disease-causing DNA variants in proline-alanine-rich ste20-related kinase (c.791dup; p.Ser265ValfsX64) and zinc finger protein 408 (ZNF408) (c.1363C>T; p.His455Tyr), the latter of which was also present in an additional Dutch FEVR family that subsequently appeared to share a common ancestor with the original family. Sequence analysis of ZNF408 in 132 additional individuals with FEVR revealed another potentially pathogenic missense variant, p.Ser126Asn, in a Japanese family. Immunolocalization studies in COS-1 cells transfected with constructs encoding the WT and mutant ZNF408 proteins, revealed that the WT and the p.Ser126Asn mutant protein show complete nuclear localization, whereas the p.His455Tyr mutant protein was localized almost exclusively in the cytoplasm. Moreover, in a cotransfection assay, the p.His455Tyr mutant protein retains the WT ZNF408 protein in the cytoplasm, suggesting that this mutation acts in a dominant-negative fashion. Finally, morpholino-induced knockdown of znf408 in zebrafish revealed defects in developing retinal and trunk vasculature, that could be rescued by coinjection of RNA encoding human WT ZNF408 but not p.His455Tyr mutant ZNF408. Together, our data strongly suggest that mutant ZNF408 results in abnormal retinal vasculogenesis in humans and is associated with FEVR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Criswick VG, Schepens CL. Familial exudative vitreoretinopathy. Am J Ophthalmol. 1969;68(4):578–594. - PubMed
-
- Canny CLB, Oliver GL. Fluorescein angiographic findings in familial exudative vitreoretinopathy. Arch Ophthalmol. 1976;94(7):1114–1120. - PubMed
-
- Boonstra FN, et al. Clinical and molecular evaluation of probands and family members with familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2009;50(9):4379–4385. - PubMed
-
- Chen ZY, et al. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet. 1993;5(2):180–183. - PubMed
-
- Gow J, Oliver GL. Familial exudative vitreoretinopathy: An expanded view. Arch Ophthalmol. 1971;86(2):150–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

