Deficits in human trisomy 21 iPSCs and neurons
- PMID: 23716668
- PMCID: PMC3683748
- DOI: 10.1073/pnas.1216575110
Deficits in human trisomy 21 iPSCs and neurons
Abstract
Down syndrome (trisomy 21) is the most common genetic cause of intellectual disability, but the precise molecular mechanisms underlying impaired cognition remain unclear. Elucidation of these mechanisms has been hindered by the lack of a model system that contains full trisomy of chromosome 21 (Ts21) in a human genome that enables normal gene regulation. To overcome this limitation, we created Ts21-induced pluripotent stem cells (iPSCs) from two sets of Ts21 human fibroblasts. One of the fibroblast lines had low level mosaicism for Ts21 and yielded Ts21 iPSCs and an isogenic control that is disomic for human chromosome 21 (HSA21). Differentiation of all Ts21 iPSCs yielded similar numbers of neurons expressing markers characteristic of dorsal forebrain neurons that were functionally similar to controls. Expression profiling of Ts21 iPSCs and their neuronal derivatives revealed changes in HSA21 genes consistent with the presence of 50% more genetic material as well as changes in non-HSA21 genes that suggested compensatory responses to oxidative stress. Ts21 neurons displayed reduced synaptic activity, affecting excitatory and inhibitory synapses equally. Thus, Ts21 iPSCs and neurons display unique developmental defects that are consistent with cognitive deficits in individuals with Down syndrome and may enable discovery of the underlying causes of and treatments for this disorder.
Keywords: cerebral cortex; developmental disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
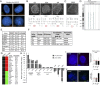

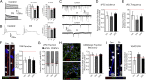
References
-
- Parker SE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. - PubMed
-
- Lejeune J, Turpin R, Gautier M. Le mongolisme, premier exemple d'aberration autosomique humaine. Annals of Genetics. 1959;1(2):41–49.
-
- Sturgeon X, Gardiner KJ. Transcript catalogs of human chromosome 21 and orthologous chimpanzee and mouse regions. Mamm Genome. 2011;22(5–6):261–271. - PubMed
-
- Letourneau A, Antonarakis SE. Genomic determinants in the phenotypic variability of Down syndrome. Prog Brain Res. 2012;197(2012):15–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

