Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens
- PMID: 23716675
- PMCID: PMC3683766
- DOI: 10.1073/pnas.1303573110
Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens
Abstract
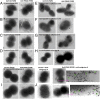
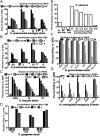
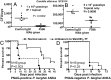
Microbial capsular antigens are effective vaccines but are chemically and immunologically diverse, resulting in a major barrier to their use against multiple pathogens. A β-(1→6)-linked poly-N-acetyl-d-glucosamine (PNAG) surface capsule is synthesized by four proteins encoded in genetic loci designated intercellular adhesion in Staphylococcus aureus or polyglucosamine in selected Gram-negative bacterial pathogens. We report that many microbial pathogens lacking an identifiable intercellular adhesion or polyglucosamine locus produce PNAG, including Gram-positive, Gram-negative, and fungal pathogens, as well as protozoa, e.g., Trichomonas vaginalis, Plasmodium berghei, and sporozoites and blood-stage forms of Plasmodium falciparum. Natural antibody to PNAG is common in humans and animals and binds primarily to the highly acetylated glycoform of PNAG but is not protective against infection due to lack of deposition of complement opsonins. Polyclonal animal antibody raised to deacetylated glycoforms of PNAG and a fully human IgG1 monoclonal antibody that both bind to native and deacetylated glycoforms of PNAG mediated complement-dependent opsonic or bactericidal killing and protected mice against local and/or systemic infections by Streptococcus pyogenes, Streptococcus pneumoniae, Listeria monocytogenes, Neisseria meningitidis serogroup B, Candida albicans, and P. berghei ANKA, and against colonic pathology in a model of infectious colitis. PNAG is also a capsular polysaccharide for Neisseria gonorrhoeae and nontypable Hemophilus influenzae, and protects cells from environmental stress. Vaccination targeting PNAG could contribute to immunity against serious and diverse prokaryotic and eukaryotic pathogens, and the conserved production of PNAG suggests that it is a critical factor in microbial biology.
Keywords: animal models; carbohydrates; immunotherapy; infectious diseases; malaria.
Conflict of interest statement
Conflict of interest statement: G.B.P., T.M.-L., M.L.G., Y.E.T., and N.E.N. are inventors of intellectual properties [human monoclonal antibody to PNAG (G.B.P. and T.M.-L.) and PNAG vaccines (G.B.P., T.M.-L., M.L.G., Y.E.T., and N.E.N.], which are licensed by Brigham and Women's Hospital to Alopexx Vaccine, LLC, and Alopexx Pharmaceuticals, LLC, entities in which G.B.P. also holds equity. As inventors of intellectual properties, these authors also have the right to receive a share of licensing-related income (royalties, fees) through Brigham and Women's Hospital from Alopexx Pharmaceuticals, LLC, and Alopexx Vaccine, LLC. G.B.P. and T.M.-L.'s interests were reviewed and are managed by the Brigham and Women's Hospital and Partners Healthcare in accordance with their conflict of interest policies. A.R. and C.P. are employees of Sanofi, Inc., which has licensed the technology for use of mAb F598 (designated as SAR279356) for treatment and prevention of human infections.
Figures



Comment in
-
Simple polysaccharides and global responses in the era of vaccinomics.Pathog Glob Health. 2013 Sep;107(6):283-4. doi: 10.1179/2047772413Z.000000000155. Pathog Glob Health. 2013. PMID: 24188238 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention (CDC) Control of infectious diseases. MMWR Morb Mortal Wkly Rep. 1999;48(29):621–629. - PubMed
-
- Toltzis P, Jacobs MR. The epidemiology of childhood pneumococcal disease in the United States in the era of conjugate vaccine use. Infect Dis Clin North Am. 2005;19(3):629–645. - PubMed
-
- Ladhani SN. Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom. Clin Ther. 2012;34(2):385–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI079293/AI/NIAID NIH HHS/United States
- U54 AI057159/AI/NIAID NIH HHS/United States
- 5R01AI079085-04/AI/NIAID NIH HHS/United States
- R21 AI046706/AI/NIAID NIH HHS/United States
- K08 AI078942/AI/NIAID NIH HHS/United States
- R01 CA154426/CA/NCI NIH HHS/United States
- R01 AI079085/AI/NIAID NIH HHS/United States
- R01 AI079293/AI/NIAID NIH HHS/United States
- AI057159/AI/NIAID NIH HHS/United States
- AI46706/AI/NIAID NIH HHS/United States
- R01 GM099537/GM/NIGMS NIH HHS/United States
- R01 AI046706/AI/NIAID NIH HHS/United States
- R01 EY016144/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

