Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states
- PMID: 23716676
- PMCID: PMC3683770
- DOI: 10.1073/pnas.1217042110
Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states
Abstract
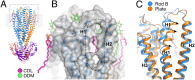
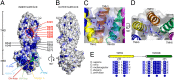
ABCB10 is one of the three ATP-binding cassette (ABC) transporters found in the inner membrane of mitochondria. In mammals ABCB10 is essential for erythropoiesis, and for protection of mitochondria against oxidative stress. ABCB10 is therefore a potential therapeutic target for diseases in which increased mitochondrial reactive oxygen species production and oxidative stress play a major role. The crystal structure of apo-ABCB10 shows a classic exporter fold ABC transporter structure, in an open-inwards conformation, ready to bind the substrate or nucleotide from the inner mitochondrial matrix or membrane. Unexpectedly, however, ABCB10 adopts an open-inwards conformation when complexed with nonhydrolysable ATP analogs, in contrast to other transporter structures which adopt an open-outwards conformation in complex with ATP. The three complexes of ABCB10/ATP analogs reported here showed varying degrees of opening of the transport substrate binding site, indicating that in this conformation there is some flexibility between the two halves of the protein. These structures suggest that the observed plasticity, together with a portal between two helices in the transmembrane region of ABCB10, assist transport substrate entry into the substrate binding cavity. These structures indicate that ABC transporters may exist in an open-inwards conformation when nucleotide is bound. We discuss ways in which this observation can be aligned with the current views on mechanisms of ABC transporters.
Keywords: ABC mitochondrial erythroid; X-ray crystallography; cardiolipin; human membrane protein structure; nucleotide complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zolnerciks JK, Andress EJ, Nicolaou M, Linton KJ. Structure of ABC transporters. Essays Biochem. 2011;50(1):43–61. - PubMed
-
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(1) Suppl:S23–S45. - PubMed
-
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
- BEP17032/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- B19456/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 092809/WT_/Wellcome Trust/United Kingdom
- BB/H000267/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I019855/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

