Nanodiscs as a therapeutic delivery agent: inhibition of respiratory syncytial virus infection in the lung
- PMID: 23717040
- PMCID: PMC3663477
- DOI: 10.2147/IJN.S39888
Nanodiscs as a therapeutic delivery agent: inhibition of respiratory syncytial virus infection in the lung
Abstract
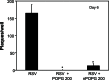
There is increasing interest in the application of nanotechnology to solve the difficult problem of therapeutic administration of pharmaceuticals. Nanodiscs, composed of a stable discoidal lipid bilayer encircled by an amphipathic membrane scaffold protein that is an engineered variant of the human Apo A-I constituent of high-density lipoproteins, have been a successful platform for providing a controlled lipid composition in particles that are especially useful for investigating membrane protein structure and function. In this communication, we demonstrate that nanodiscs are effective in suppressing respiratory syncytial viral (RSV) infection both in vitro and in vivo when self-assembled with the minor pulmonary surfactant phospholipid palmitoyloleoylphosphatidylglycerol (POPG). Preparations of nanodiscs containing POPG (nPOPG) antagonized interleukin-8 production from Beas2B epithelial cells challenged by RSV infection, with an IC50 of 19.3 μg/mL. In quantitative in vitro plaque assays, nPOPG reduced RSV infection by 93%. In vivo, nPOPG suppressed inflammatory cell infiltration into the lung, as well as IFN-γ production in response to RSV challenge. nPOPG also completely suppressed the histopathological changes in lung tissue elicited by RSV and reduced the amount of virus recovered from lung tissue by 96%. The turnover rate of nPOPG was estimated to have a halftime of 60-120 minutes (m), based upon quantification of the recovery of the human Apo A-I constituent. From these data, we conclude that nPOPG is a potent antagonist of RSV infection and its inflammatory sequelae both in vitro and in vivo.
Keywords: anti-viral; innate immunity; nanodiscs; phospholipids; therapeutic delivery.
Figures








References
-
- Bayburt TH, Grinkova YV, Sligar SG. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Letters. 2002;2(8):853–856.
-
- Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J Am Chem Soc. 2004;126(11):3477–3487. - PubMed
-
- Atkinson D, Small DM. Recombinant lipoproteins: implications for structure and assembly of native lipoproteins. Annu Rev Biophys Biophys Chem. 1986;15:403–456. - PubMed
-
- Atkinson D, Smith HM, Dickson J, Austin JP. Interaction of apoprotein from porcine high-density lipoprotein with dimyristoyl lecithin. 1. The structure of the complexes. Eur J Biochem. 1976;64(2):541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

