Chemodiversity in Selaginella: a reference system for parallel and convergent metabolic evolution in terrestrial plants
- PMID: 23717312
- PMCID: PMC3650682
- DOI: 10.3389/fpls.2013.00119
Chemodiversity in Selaginella: a reference system for parallel and convergent metabolic evolution in terrestrial plants
Abstract
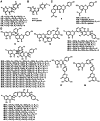
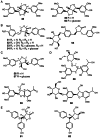
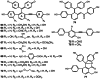
Early plants began colonizing the terrestrial earth approximately 450 million years ago. Their success on land has been partially attributed to the evolution of specialized metabolic systems from core metabolic pathways, the former yielding structurally and functionally diverse chemicals to cope with a myriad of biotic and abiotic ecological pressures. Over the past two decades, functional genomics, primarily focused on flowering plants, has begun cataloging the biosynthetic players underpinning assorted classes of plant specialized metabolites. However, the molecular mechanisms enriching specialized metabolic pathways during land plant evolution remain largely unexplored. Selaginella is an extant lycopodiophyte genus representative of an ancient lineage of tracheophytes. Notably, the lycopodiophytes diverged from euphyllophytes over 400 million years ago. The recent completion of the whole-genome sequence of an extant lycopodiophyte, S. moellendorffii, provides new genomic and biochemical resources for studying metabolic evolution in vascular plants. 400 million years of independent evolution of lycopodiophytes and euphyllophytes resulted in numerous metabolic traits confined to each lineage. Surprisingly, a cadre of specialized metabolites, generally accepted to be restricted to seed plants, have been identified in Selaginella. Initial work suggested that Selaginella lacks obvious catalytic homologs known to be involved in the biosynthesis of well-studied specialized metabolites in seed plants. Therefore, these initial functional analyses suggest that the same chemical phenotypes arose independently more commonly than anticipated from our conventional understanding of the evolution of metabolism. Notably, the emergence of analogous and homologous catalytic machineries through convergent and parallel evolution, respectively, seems to have occurred repeatedly in different plant lineages.
Keywords: Selaginella; chemodiversity; convergent evolution; parallel evolution; specialized metabolism.
Figures





Similar articles
-
Parallels in lignin biosynthesis: A study in Selaginella moellendorffii reveals convergence across 400 million years of evolution.Commun Integr Biol. 2008;1(1):20-2. doi: 10.4161/cib.1.1.6466. Commun Integr Biol. 2008. PMID: 19704782 Free PMC article.
-
Global transcriptome analysis reveals extensive gene remodeling, alternative splicing and differential transcription profiles in non-seed vascular plant Selaginella moellendorffii.BMC Genomics. 2017 Jan 25;18(Suppl 1):1042. doi: 10.1186/s12864-016-3266-1. BMC Genomics. 2017. PMID: 28198676 Free PMC article.
-
Phosphoproteomic analysis of the non-seed vascular plant model Selaginella moellendorffii.Proteome Sci. 2014 Mar 17;12:16. doi: 10.1186/1477-5956-12-16. eCollection 2014. Proteome Sci. 2014. PMID: 24628833 Free PMC article.
-
Convergent evolution in plant specialized metabolism.Annu Rev Plant Biol. 2011;62:549-66. doi: 10.1146/annurev-arplant-042110-103814. Annu Rev Plant Biol. 2011. PMID: 21275647 Review.
-
Selaginella and 400 million years of separation.Annu Rev Plant Biol. 2009;60:223-38. doi: 10.1146/annurev.arplant.59.032607.092851. Annu Rev Plant Biol. 2009. PMID: 19575581 Review.
Cited by
-
Candidate genes of flavonoid biosynthesis in Selaginella bryopteris (L.) Baker identified by RNA-Seq.Funct Integr Genomics. 2018 Sep;18(5):505-517. doi: 10.1007/s10142-018-0603-2. Epub 2018 Apr 18. Funct Integr Genomics. 2018. PMID: 29666977
-
Accumulation of Salicylic Acid and Related Metabolites in Selaginella moellendorffii.Plants (Basel). 2022 Feb 8;11(3):461. doi: 10.3390/plants11030461. Plants (Basel). 2022. PMID: 35161442 Free PMC article.
-
The ancient CYP716 family is a major contributor to the diversification of eudicot triterpenoid biosynthesis.Nat Commun. 2017 Feb 6;8:14153. doi: 10.1038/ncomms14153. Nat Commun. 2017. PMID: 28165039 Free PMC article.
-
Ipecac alkaloid biosynthesis in two evolutionarily distant plants.Nat Chem Biol. 2025 Jun 3. doi: 10.1038/s41589-025-01926-z. Online ahead of print. Nat Chem Biol. 2025. PMID: 40461725
-
The Origin and Evolution of Plant Flavonoid Metabolism.Front Plant Sci. 2019 Aug 2;10:943. doi: 10.3389/fpls.2019.00943. eCollection 2019. Front Plant Sci. 2019. PMID: 31428108 Free PMC article. Review.
References
-
- Abe I., Morita H. (2010). Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat. Prod. Rep. 27 809–838 - PubMed
-
- Austin M. B., Izumikawa M., Bowman M. E., Udwary D. W., Ferrer J. L., Moore B. S., et al. (2004). Crystal structure of a bacterial type III polyketide synthase and enzymatic control of reactive polyketide intermediates. J. Biol. Chem. 279 45162–45174 - PubMed
-
- Austin M. B., Noel J. P. (2003). The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 20 79–110 - PubMed
-
- Bais H. P., Vepachedu R., Lawrence C. B., Stermitz F. R., Vivanco J. M. (2003). Molecular and biochemical characterization of an enzyme responsible for the formation of hypericin in St. John's wort (Hypericum perforatum L.). J. Biol. Chem. 278 32413–32422 - PubMed
-
- Banks J. A. (2009). Selaginella and 400 million years of separation. Annu. Rev. Plant Biol. 60 223–238 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

