Staphylococcus aureus-induced G2/M phase transition delay in host epithelial cells increases bacterial infective efficiency
- PMID: 23717407
- PMCID: PMC3662696
- DOI: 10.1371/journal.pone.0063279
Staphylococcus aureus-induced G2/M phase transition delay in host epithelial cells increases bacterial infective efficiency
Abstract
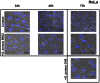
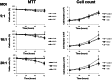
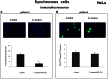
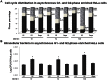
Staphylococcus aureus is a highly versatile, opportunistic pathogen and the etiological agent of a wide range of infections in humans and warm-blooded animals. The epithelial surface is its principal site of colonization and infection. In this work, we investigated the cytopathic effect of S. aureus strains from human and animal origins and their ability to affect the host cell cycle in human HeLa and bovine MAC-T epithelial cell lines. S. aureus invasion slowed down cell proliferation and induced a cytopathic effect, resulting in the enlargement of host cells. A dramatic decrease in the number of mitotic cells was observed in the infected cultures. Flow cytometry analysis revealed an S. aureus-induced delay in the G2/M phase transition in synchronous HeLa cells. This delay required the presence of live S. aureus since the addition of the heat-killed bacteria did not alter the cell cycle. The results of Western blot experiments showed that the G2/M transition delay was associated with the accumulation of inactive cyclin-dependent kinase Cdk1, a key inducer of mitosis entry, and with the accumulation of unphosphorylated histone H3, which was correlated with a reduction of the mitotic cell number. Analysis of S. aureus proliferation in asynchronous, G1- and G2-phase-enriched HeLa cells showed that the G2 phase was preferential for bacterial infective efficiency, suggesting that the G2 phase delay may be used by S. aureus for propagation within the host. Taken together, our results divulge the potential of S. aureus in the subversion of key cellular processes such as cell cycle progression, and shed light on the biological significance of S. aureus-induced host cell cycle alteration.
Conflict of interest statement
Figures





References
-
- Edwards AM, Massey RC (2011) How does Staphylococcus aureus escape the bloodstream? Trends Microbiol 19: 184–190. - PubMed
-
- Fitzgerald JR (2012) Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol 20: 192–198. - PubMed
-
- Steeneveld W, van Werven T, Barkema HW, Hogeveen H (2011) Cow-specific treatment of clinical mastitis: An economic approach. J Dairy Sci 94: 174–188. - PubMed
-
- Bougarn S, Cunha P, Gilbert FB, Harmache A, Foucras G, et al. (2011) Staphylococcal-associated molecular patterns enhance expression of immune defense genes induced by IL-17 in mammary epithelial cells. Cytokine 56: 749–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

