Pancreatic islet xenograft survival in mice is extended by a combination of alpha-1-antitrypsin and single-dose anti-CD4/CD8 therapy
- PMID: 23717456
- PMCID: PMC3661573
- DOI: 10.1371/journal.pone.0063625
Pancreatic islet xenograft survival in mice is extended by a combination of alpha-1-antitrypsin and single-dose anti-CD4/CD8 therapy
Abstract
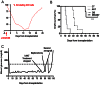
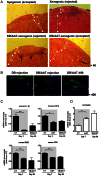
Clinical pancreatic islet transplantation is under evaluation for the treatment of autoimmune diabetes, yet several limitations preclude widespread use. For example, there is a critical shortage of human pancreas donors. Xenotransplantation may solve this problem, yet it evokes a rigorous immune response which can lead to graft rejection. Alpha-1-antitrypsin (AAT), a clinically available and safe circulating anti-inflammatory and tissue protective glycoprotein, facilitates islet alloimmune-tolerance and protects from inflammation in several models. Here, we examine whether human AAT (hAAT), alone or in combination with clinically relevant approaches, achieves long-term islet xenograft survival. Rat-to-mouse islet transplantation was examined in the following groups: untreated (n = 6), hAAT (n = 6, 60-240 mg/kg every 3 days from day -10), low-dose co-stimulation blockade (anti-CD154/LFA-1) and single-dose anti-CD4/CD8 (n = 5-7), either as mono- or combination therapies. Islet grafting was accompanied by blood glucose follow-up. In addition, skin xenografting was performed in order to depict responses that occur in draining lymph nodes. According to our results hAAT monotherapy and hAAT/anti-CD154/LFA-1 combined therapy, did not delay rejection day (11-24 days untreated vs. 10-22 day treated). However, host and donor intragraft inflammatory gene expression was diminished by hAAT therapy in both setups. Single dose T-cell depletion using anti-CD4/CD8 depleting antibodies, which provided 14-15 days of reduced circulating T-cells, significantly delayed rejection day (28-52 days) but did not achieve graft acceptance. In contrast, in combination with hAAT, the group displayed significantly extended rejection days and a high rate of graft acceptance (59, 61, >90, >90, >90). In examination of graft explants, marginal mononuclear-cell infiltration containing regulatory T-cells predominated surviving xenografts. We suggest that temporal T-cell depletion, as in the clinically practiced anti-thymocyte-globulin therapy, combined with hAAT, may promote islet xenograft acceptance. Further studies are required to elucidate the mechanism behind the observed synergy, as well as the applicability of the approach for pig-to-human islet xenotransplantation.
Conflict of interest statement
Figures


References
-
- de Kort H, de Koning EJ, Rabelink TJ, Bruijn JA, Bajema IM (2011) Islet transplantation in type 1 diabetes. BMJ 342: d217. - PubMed
-
- Sprangers B, Waer M, Billiau AD (2008) Xenotransplantation: where are we in 2008? Kidney Int 74: 14–21. - PubMed
-
- Jones PM, Courtney ML, Burns CJ, Persaud SJ (2008) Cell-based treatments for diabetes. Drug Discov Today 13: 888–893. - PubMed
-
- Rayat GR, Johnson ZA, Beilke JN, Korbutt GS, Rajotte RV, et al. (2003) The degree of phylogenetic disparity of islet grafts dictates the reliance on indirect CD4 T-cell antigen recognition for rejection. Diabetes 52: 1433–1440. - PubMed
-
- Koulmanda M, Laufer TM, Auchincloss H Jr, Smith RN (2004) Prolonged survival of fetal pig islet xenografts in mice lacking the capacity for an indirect response. Xenotransplantation 11: 525–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

